Exploring cannabis use by patients with multiple sclerosis in a state where cannabis is legal
Laura Weinkle M.S. , Christopher H. Domen Ph.D. , Ian Shelton B.A. ,
Stefan Sillau Ph.D. , Kavita Nair Ph.D. , Enrique Alvarez M.D., Ph.D. , Exploring cannabis use by pa-
tients with multiple sclerosis in a state where cannabis is legal, Multiple Sclerosis and Related Disorders
(2018), doi: https://doi.org/10.1016/j.msard.2018.11.022
Highlights:
38% percent of Multiple Sclerosis patients surveyed were current cannabis users.
74% of Multiple Sclerosis patients would personally consider using cannabis.
Cannabis users reported higher median disability on the PDDS than non-users.
53% of cannabis users had limited exposure to cannabis prior to their diagnosis.
Cannabis use may be causing reduced use of conventional pharmaceutical therapies.
Abstract
Background:
Studies suggest cannabis may improve symptoms like pain and muscle spasticity in patients
with multiple sclerosis (PwMS). Despite cannabis’ new-found legality and availability, few studies have
explored the profile of PwMS cannabis users and characteristics of their use, particularly in a state where
cannabis is legal both for recreational and medicinal use. The purpose of the current study was to evaluate
cannabis use among PwMS at a large academic multiple sclerosis (MS) clinic, specifically: 1) prevalence;
2) products used (e.g., cannabidiol vs Δ9-tetrahydocannabinol) 3) symptom treatment; and 4) patient
characteristics.
Methods:
PwMS completed questions assessing personal opinions about cannabis use, characteristics of
cannabis use, MS history, diagnosis, sociodemographic details, as well as the self-reported disability-
Patient Determined Disease Steps (PDDS), overall quality of life-the Patient Reported Outcome Measure
Information System (PROMIS-10), and cognition-the Neuro-QoL ACGC v1.0 measures.
Results:
Thirty-eight percent (n = 96) of PwMS were current Cannabis users (CUs). Although there were
no sociodemographic or clinical differences (p ≤ 0.05) between CUs and Non-Cannabis users (NUs), CUs
had significantly higher median disability compared to NUs (PDDS = 2 vs. 1; p = 0.02). Among CUs,
57% categorized their use as strictly medicinal. CUs reported using cannabis most often for pain and
insomnia/poor sleep and experienced greater than 60% benefit/relief from cannabis use. Over 90% of
respondents desire more research on cannabis for MS, and 74% indicated they would consider using
cannabis for their MS.
Conclusion:
As cannabis legalization has impacted the variety of cannabis products available, there
appears to be growing numbers of PwMS using cannabis, with this study’s CUs reporting use of highly
efficacious products with minimal side-effects.
Introduction
The effort to legalize cannabis (marijuana) has gathered substantial momentum, with 30
states and the District of Columbia having passed legislation legalizing cannabis in some form,
with legalization of recreational use in nine states. As the legal status of cannabis has shifted, the
safety and efficacy of cannabis as a treatment option has become an increasingly pertinent topic.
Debate concerning the medicinal usefulness of cannabis largely persists because of the plant’s
complexity. The cannabis plant contains over 100 cannabinoids, though the two most well-
known are Δ9-tetrahydocannabinol (THC) and cannabidiol (CBD) (Atakan, 2012; Andre et al.,
2016). Cannabinoids exert their effects primarily via the endogenous cannabinoid system
through CB1 and CB2 receptors. While CB1 receptors are primarily found in the central nervous
system, CB2 receptors are mostly found in the periphery in immune cells. THC’s psychoactive
effects are principally mediated by agonist effects at CB1 receptors. Conversely, CBD is thought
to function more as an antagonist at CB1 and CB2 receptors and act to inhibit immune cells
(Bogs et al, 2018; Booz, 2011). The anti-inflammatory, as well as analgesic, properties
associated with CBD have driven its reputation as non-psychoactive and medicinally useful.
Indeed, evidence from clinical studies support the therapeutic efficacy of cannabis and
cannabinoid-based medicines for the treatment of a myriad of diseases and associated symptoms
(Giacoppo et al., 2014; Koppel et al., 2014; Belendiuk et al., 2015; Fife et al., 2015).
In patients with Multiple Sclerosis (PwMS), cannabis products have been shown to
provide relief from symptoms including central pain, muscle spasticity/tightness, and painful
muscle spasms (Svendsen et al., 2004; Wade et al., 2004; Zajicek et al., 2005; Wade et al., 2006;
Zajicek and Apostu, 2011; Corey-Bloom et al., 2012; Giacoppo et al., 2014; Koppel et al., 2014;
Belendiuk et al., 2015; Fife et al., 2015; Rice and Cameron, 2018). These clinical studies are
bolstered by over 20 years of observational data from questionnaires systematically assessing
perceptions of cannabis, extent of use, and perceived impact on MS symptoms (Consroe et al.,
1997; Linassi and Hader, 2003; Page et al., 2003; Clark et al., 2004; Chong et al., 2006; Cofield
et al., 2017; Brenton et al., 2018; Gupta et al., 2018). With greater access to cannabis products, a
growing body of scientific data, and anecdotal reports supporting the potential benefit of
cannabis, there is increased interest and use of cannabis products for MS symptom management.
Indeed, 16% of PwMS currently use cannabis for MS, and 50-90% would consider using it if it
were legal and more evidence supporting its safety and efficacy available (Chong et al., 2006;
Cofield et al., 2017; Rudroff and Honce, 2017; Rice and Cameron, 2018).
Certainly, the above-mentioned studies highlight cannabis’ potential benefit for MS
treatment and the growing acceptance of its use. However, cannabis’ shift in legality has done
more than simply make cannabis more available, it has significantly impacted the variety of
products available. For example, cannabis may be consumed by combusting/smoking the flower,
vaporizing the flower or processed oil, prepared edibles, etc. Also, cannabis products are
available with varying cannabinoid profiles, most prominently with varying ratios of THC to
CBD. Thus, the question is no longer simply whether PwMS are using cannabis, but, rather, what
specific products are being used by PwMS. While prior studies have explored methods of
ingestion (e.g., Banwell et al., 2016; Brenton et al., 2018), none has explored the cannabinoid
profiles of products currently being used by PwMS. Furthermore, no prior studies have examined
patterns of usage associated with self-identification as either a recreational or medicinal cannabis
user (CU). This manner of self-identification predicts different patterns of use. It seems likely
that medicinal CUs want to maximize treatment effects but limit side-effects, whereas recreational CUs seek to experience the psyachoactive effects. Thus, it is critical to explore the
profile of CUs and characteristics of cannabis use among PwMS in a state where cannabis is
medicinally and recreationally legal. To that end, this study explored 1) the perceptions of
cannabis use for MS symptom management; 2) the extent and characteristics of cannabis use, in
part focusing on the cannabinoid profiles of products used and differences in use between self-
identified medicinal or recreational (or both) CUs; and 3) the impact of cannabis on symptoms
among PwMS.
Material and methods
Setting and recruitment
This study was approved by the Colorado Multiple Institutional Review Board. From
October 2017 to April 2018, PwMS at the Rocky Mountain Multiple Sclerosis Center at the
University of Colorado (RMMSC) were randomly identified and approached during a routine
clinic visit to participate in an anonymous, voluntary digital survey administered on a tablet
computer. Potential participants were identified by record review prior to being approached for
survey completion as being established patients previously diagnosed with MS by a Board
Certified Neurologist at the RMMSC according to McDonald criteria (Thompson et al., 2017).
Respondents confirmed inclusion criteria: MS diagnosis and between 18-89 years of age old.
Incomplete surveys were excluded (i.e., multiple sequential questions were incomplete, and
survey was not submitted as complete.)
Survey tool and design
The digital survey tool was developed by clinicians at the University of Colorado through
consultation with cannabis industry personnel and patients in the form of one-on-one type
discussions, email exchanges, and a focus group led by one of the authors. The survey was
composed of four sections. Section 1 explored personal opinions about cannabis use (e.g.,
perceived benefit or harm of cannabis for MS). Section 2 consisted of questions exploring the
characteristics of each patient’s cannabis use (e.g., frequency of use, preferred method of
consumption). Due to skip-logic survey design, only respondents who endorsed personally
considering using cannabis for their MS in Section 1 were administered Section 2 (all
respondents were administered Sections 1, 3, and 4). Section 3 was comprised of questions
related to MS history/diagnosis and sociodemographic details. Finally, Section 4 included three
Patient Reported Health Outcomes (PROs) measures: 1) Patient Disease Determined Steps
(PDDS), which assesses respondents’ walking ability (a proxy for self-reported disability;
Learmonth et al., 2013); 2) Patient-Reported Outcomes Measurement Information System
(PROMIS-10) Global Health scale, which measures respondents’ health-related quality of life
through two composite measures - physical health and mental health (Hays et al., 2009); and 3)
Quality of Life in Neurological Disorders Applied Cognition - General Concerns Short Form
version 1.0 (Neuro-QoL ACGC), which evaluates perceived concerns with memory, attention,
and decision-making (National Institute of Neurological Disorders and Stroke, 2015).
Statistical analysis
SAS® software, Version 9.4, was used for all statistical analyses. Chi-square/Fisher’s
exact association test were used for comparing categorical outcomes between groups, and T-
tests/ANOVA for comparing continuous or scale outcomes between groups. The PROMIS-10
and Neuro-QoL ACGC were scored according to the developers’ instructions and T scores are
reported; higher T score indicates greater satisfaction with physical and mental health, and fewer
cognitive concerns, respectively. For PDDS, raw score was reported, and lower score indicates
less disability. Written comments were provided by 85 Cannabis users and coded based on
keywords or phrases, then themes identified. The number of respondents were noted for each
coded keywords or phrases in the comments to determine frequency but without amplifying the
numbers for those individuals mentioning certain keywords more than once. A visualization of
these qualitative data was created in Word Cloud Generator – Jason Davies© (London, UK).
Results
Patient population
Two hundred and fifty-one patients completed the survey. Table 1 shows
sociodemographic characteristics, clinical characteristics, and PRO measures. Non-Cannabis
users (NUs; 62%) were identified if respondents indicated they 1) would not personally consider
using cannabis for their MS or 2) would personally consider using cannabis for MS but denied
cannabis use in the past 12-months. Respondents were identified as Current CUs (38%) if they
endorsed 1) would personally consider using cannabis to manage their MS and 2) had used
cannabis in the past 12-months. There were no sociodemographic or clinical differences between
NUs and CUs (all p-values > 0.05). CUs had significantly higher median disability (PDDS = 2)
than NUs (PDDS = 1; p < 0.05). No differences were observed on other PROs measures (all p- values > 0.05).
| CHARACTERISTIC/MEASURE | All N=251 | Non- Cannabis N=155 | Cannabis N=96 | p-value* |
| AGE at survey, mean (±SD) | 45 (±13) | 46 (± 13) | 44 (± 13) | 0.12 |
| AGE at MS onset, mean (±SD) | 34 (±12) | 35 (± 12) | 32 (± 13) | 0.20 |
| SEX % | ||||
| Female | 76 | 78 | 74 | 0.43 |
| RACE % | ||||
| White | 89 | 90 | 87 | 0.66 |
| Black | 6 | 6 | 6 | |
| ETHNICITY % | 0.50 | |||
| Hispanic/Latino or Other Spanish origin | 9 | 10 | 7 | |
| STATE OF RESIDENCE % | 0.69 | |||
| CO | 92 | 90 | 95 | |
| KSǂ, MIᶲ, MTᶲ, NEᶴ, NMᶲ, NV§, SDᶴ, TNǂ, UTǂ, WYǂ | 8 | 10 | 5 | |
| MARITAL STATUS % | 0.24 | |||
| Single | 17 | 16 | 20 | |
| Married/Cohabitating | 67 | 70 | 60 | |
| Divorced/Separated | 15 | 13 | 17 | |
| Widowed | 2 | 1 | 3 | |
| EDUCATION LEVEL % | 0.50 | |||
| High School Diploma/GED or Less | 25 | 23 | 28 | |
| Bachelor's or Technical College/Associates Degree | 53 | 55 | 48 | |
| Graduate Degree (Master's, JD, Doctoral) | 23 | 22 | 23 | |
| ANNUAL HOUSEHOLD INCOME % | 0.09 | |||
| $30,000 or less | 26 | 21 | 35 | |
| $30,001-$60,000 | 16 | 18 | 13 | |
| $60,001 - $150,000 | 48 | 50 | 43 | |
| $150,000 | 11 | 11 | 10 | |
| EMPLOYMENT STATUS % | 0.07 | |||
| Employed** | 59 | 64 | 51 | |
| Unable to work | 26 | 20 | 34 | |
| Out of work | 4 | 3 | 4 | |
| Retired | 12 | 13 | 10 | |
| MS relapse, ever % | 0.44 | |||
| Yes | 72 | 71 | 74 | |
| Progressively worsening disability, 12-mo % | 0.31 | |||
| Yes | 34 | 30 | 39 | |
| Present MS Symptom Control % | 0.27 | |||
| Very well controlled | 30 | 23 | 35 | |
| Moderately well controlled | 32 | 31 | 33 | |
| Somewhat controlled | 18 | 23 | 15 | |
| Minimally controlled | 5 | 7 | 5 | |
| Not controlled | 2 | 3 | 2 | |
| No current symptoms | 12 | 13 | 11 | |
| PDDS, median | 2 | 1 | 2 | |
| PROMIS-10, mean (± SD) | 0.02 | |||
| Physical Health*** | 34 (± 7) | 34 (± 7) | 33 (± 6) | 0.22 |
| Mental Health*** | 46 (± 8) | 47 (± 8) | 45 (± 8) | 0.13 |
| Neuro-QoL ACGC, mean (± SD)*** | 44 (± 9) | 45 (± 9) | 44 (± 9) | 0.24 |
Table 1. Sociodemographic characteristics, Multiple Sclerosis (MS) clinical characteristics and Patient Reported Outcome (PROs) measures of All respondents, Non-Cannabis users and Cannabis users. Medicinal and recreational cannabis use legalized (NV); ǂ = Cannabis products with limited THC content legalized for medicinal use (KS, TN, UT, WY); ᶲ = Medicinal cannabis use legalized (MI, MT, NM); ᶴ =Cannabis use illegal (NE, SD). **Employed: Full-time; Part-time; Self-employed; Homemaker; Student.
***T scores. PDDS= Patient Determined Disease Steps; PROMIS-10= Patient-Reported Outcomes
Measurement Information System-10; ACGC = Applied Cognition – General Concerns.
Perceptions of cannabis
All respondents’ opinions on cannabis use and its effects on MS are reported in Table 2.
Most respondents indicated a desire for more research on cannabis for MS and 74% would
consider using cannabis to manage their MS. Pain, anxiety, and muscle spasm were the most
common MS symptoms selected by respondents indicating a belief that cannabis had at least
some benefit (60%) or both benefit and harm (6%) on MS symptoms. Slowed thinking,
decreased attention, and memory problems were the most common side-effects selected by
respondents indicating they perceived cannabis to be harmful (2%) or have both benefit and
harm on MS symptoms.
Table 2. Opinion on use of cannabis and its effects on MS from All respondents. A. General perceptions
of cannabis use for MS symptoms. B. Symptoms perceived to benefit from cannabis use. B. Side-effects
of cannabis use perceived to be concerning.
| GENERAL PERCEPTIONS | All N=251 | PERCEIVED SYMPTOMS | N=166** (%) | PERCEIVED SIDE-EFFECTS | N=21** (%) |
| More research % | |||||
| Yes | 95 | ||||
| Effect of cannabis on MS symptom | |||||
| Absolutely beneficial | 28 | Pain | 78 | Slowed | 90 |
| Some benefit | 32 | Anxiety | 76 | Decreased | 62 |
| No effect | 2 | Muscle spasm | 68 | Memory | 48 |
| Absolutely harmful | 2 | Spasticity/Musc | 63 | Sleepiness | 48 |
| Both beneficial and harmful | 6 | Insomnia/Poor | 61 | Anxiety | 43 |
| I don't know | 29 | Appetite | 54 | Fatigue | 38 |
| Nausea | 45 | Hallucination | 38 | ||
| Tremor | 30 | Depression | 33 | ||
| Fatigue | 20 | Weight gain | 33 | ||
| Consider using to manage MS % | Decrease MS | 17 | Headache | 29 | |
| Yes | 74 | Attention | 13 | Shaking | 19 |
| Sexual | 10 | Increase | 14 | ||
| Memory | 7 | Stomach | 14 | ||
| Vision | 7 | Sweating | 10 | ||
| Bowel/Bladder | 7 | Chills | 5 | ||
| None | 1 | Weight loss | 0 | ||
| None | 0 |
Characteristics of cannabis use
Table 3 shows characteristics of cannabis use. Combusting/smoking (30%), vaporizing
(22%) and prepared edibles (22%) were the preferred methods of using cannabis. Additionally,
79% of users obtain cannabis from a dispensary, with 43% of CUs spending less than $25
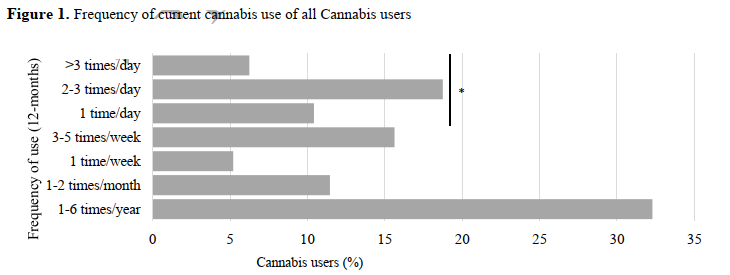
monthly. Furthermore, as is shown in Figure 1, 35% of CUs reported using at least once per day
or more, and 62% of these respondents endorsed using cannabis like a prescription drug (e.g.
bedtime, twice daily, after meals…).
Table 3. Cannabis history and characteristic of current cannabis use of all Cannabis users Cannabis history and characteristic of current cannabis use of all Cannabis users
| CHARACTERISTICS | N=96 (%) |
| Number of uses prior to MS diagnosis | 23 |
| Never | 30 |
| Less than 15 times | 19 |
| 15 - 100 times | 15 |
| 100-1000 times | 14 |
| 1000 times | 30 |
| Preferred method of use Combust/Smoke | |
| Vaporize/Vape pen | 22 |
| Prepared edibles | 22 |
| Sublingual/Tincture/Oil | 12 |
| Transdermal | 6 |
| Capsule | 8 |
| Cannabis product preference | |
| CBD only | 20 |
| THC only | 9 |
| Similar quantities of CBD & THC | 49 |
| No | 8 |
| I don't know | 14 |
| Self-categorization of use | |
| Medicinal | 57 |
| Recreational | 8 |
| Both | 35 |
| Medical Marijuana cardholder | |
| Yes | 31 |
| Preferred method of obtaining | |
| Dispensary | 79 |
| Personally grow | 5 |
| Other | 16 |
| Monthly cost for symptom treatment | |
| <$25 | 43 |
| $25-$49 | 24 |
| $50-$99 | 16 |
| $100-$199 | 13 |
| $200-$500 | 4 |
THC = Tetrahydrocannabinol
Figure 1. Frequency of current cannabis use of all Cannabis users
whether they used Cannabis like a prescription drug (e.g. bedtime, twice daily, after meals…?)
Characteristics of CUs were further explored by grouping users by their cannabis product
preference (CBD only vs. THC only and similar quantities of CBD and THC), as well as self-
categorization of use (Strictly medicinal vs. Not strictly medicinal - recreational and both
recreational and medicinal use). Of users with a preference for CBD only products, 90% self-
categorized their use as ―Strictly medicinal‖, while 52% of users with a preference for THC only
or similar quantities of CBD and THC products endorsed strictly medicinal cannabis use (p <
0.05). CBD only users were significantly less likely to: combust cannabis (0%; p < 0.05), possess
a medical marijuana card (16%; p < 0.05), and spend more than $25 monthly on cannabis for
symptom treatment (68%; p < 0.05; further group data regarding cannabinoid profile preference
is available in table form as an e-component). As is shown in Table 4, at the p < 0.05 level, there
were statistically significant differences between strictly medicinal and not strictly medicinal
CUs with respect to their age at survey and MS diagnosis, the number of uses prior to their MS
diagnosis, preferred method of using cannabis products, and cannabis product preference.
Strictly medicinal users also reported significantly greater median disability (PDDS = 3) than
their counterparts (PDDS = 1), and significantly lower mean physical health (T score = 32 ± 6)
than not strictly medicinal users (T score = 35 ± 6).
Table 4. Characteristics of Cannabis users grouped by self-categorization of current cannabis use (Strictly medicinal vs. Not strictly medicinal).
| CHARACTERISTICS | Strictly medicinal N = 54 | Not strictly medicinal N = 41 | p-value |
| AGE at survey, mean ( ± SD) | 46 ( ± 12) | 41 ( ± 12) | 0.03 |
| AGE at MS onset, mean (± SD) | 36 ( ± 12) | 30 ( ± 10) | 0.01 |
| Number of uses prior to MS diagnosis % | 0.04 | ||
| Never | 33 | 10 | |
| Less than 15 times | 31 | 29 | |
| 15 - 100 times | 17 | 20 | |
| 100-1000 times | 9 | 22 | |
| >1000 times | 9 | 20 | |
| Frequency of use (12-mo) % | 0.43 | ||
| 1-6 times/year | 30 | 34 | |
| 1-2 times/month | 13 | 10 | |
| 1 time/week | 7 | 2 | |
| 3-5 times/week | 11 | 22 | |
| 1 time/day | 13 | 7 | |
| 2-3 times/day | 22 | 15 | |
| 3 times/day | 4 | 10 | |
| Preferred method of use % | <0.0001 | ||
| Combust/Smoke | 9 | 56 | |
| Vaporize/Vape pen | 28 | 15 | |
| Prepared edibles | 25 | 20 | |
| Sublingual/Tincture/Oil | 17 | 5 | |
| Capsule | 8 | 5 | |
| Transdermal (Patch) | 13 | 0 | |
| Cannabis product preference % | 0.0002 | ||
| CBD only | 31 | 5 | |
| THC only | 2 | 20 | |
| Similar quantities of CBD & THC | 52 | 46 | |
| No | 4 | 15 | |
| I don't know | 11 | 15 | |
| Medical Marijuana cardholder % | 0.19 | ||
| Yes | 37 | 24 | |
| Preferred method of obtaining % | 0.29 | ||
| Dispensary | 81 | 76 | |
| Personally grow | 7 | 2 | |
| Other | 11 | 22 | |
| Monthly cost for symptom treatment % | 0.45 | ||
| <$25 | 48 | 37 | |
| $25-$49 | 20 | 29 | |
| $50-$99 | 15 | 17 | |
| $100-$199 | 15 | 10 | |
| $200-$500 | 2 | 7 | |
| Feel comfortable discussing cannabis for MS with physician | 0.61 | ||
| Yes | 89 | 85 | |
| Have discussed cannabis for MS with physician | 0.48 | ||
| Yes | 54 | 46 | |
| PDDS, median | 3 | 1 | |
| PROMIS-10, mean (± SD) | 0.01 | ||
| Physical Health T-score | 32 (± 6) | 35 (± 6) | 0.02 |
| Mental Health T-score | 46 (± 8) | 46 (± 9) | 0.94 |
| Neuro-QoL ACGC, T score mean (± SD) | 43 (± 9) | 44 (± 8 ) | 0.53 |
Benefits/risks of cannabis use
The most and second most common symptoms CUs reported using cannabis for were pain,
insomnia/poor sleep, and spasticity/muscle tightness, shown in Table 5. In turn, the most and
second most bothersome/worrisome side-effects selected by CUs were slowed thinking, weight
gain, and decreased attention, shown in Table 5. Notably, however, CUs selected ―none‖ most
often from the side-effects lists. CUs who used CBD only products (n=19) endorsed using them
to treat the following symptoms most commonly: ―Spasticity/Muscle tightness‖ (42%), ―Pain‖
(32%), ―Insomnia/Poor sleep‖ (21%), and ―Muscle spasm‖ (21%). CUs who used THC only
products and products with similar quantities of CBD and THC (n=56) endorsed using them to
treat the following symptoms most commonly: ―Pain‖ (52%), ―Insomnia/Poor sleep‖ (32%),
―Spasticity/Muscle tightness‖ (23%), and ―Muscle spasm‖ (20%).
Table 5. Symptoms and side-effects identified by Cannabis users.
| SYMPTOMS | All CUs* | Perceived symptom impact on QoL** | Perceived effect of cannabis on symptom** | SIDE- EFFECTS | All CUs* | Perceived severity of side-effect on QoL** |
| Pain | 39 | 65 (± 26) | 73 (± 22) | None | 75 | |
| Insomnia/Poor sleep | 27 | 65 (± 29) | 73 (± 25) | Slowed thinking | 10 | 44 (± 26) |
| None | 26 | Weight gain | 10 | 40 (± 23) | ||
| Spasticity/Muscle tightness | 25 | 54 (± 27) | 66 (± 29) | Decreased attention | 7 | 29 (± 13) |
| Anxiety | 21 | 49 (± 25) | 60 (± 14) | Fatigue | 6 | 47 (± 24) |
| Muscle spasm | 18 | 55 (± 33) | 70 (± 25) | Sleepiness | 6 | 55 (± 25) |
| Fatigue | 8 | 60 (± 25) | 46 (± 33) | Anxiety | 4 | 53 (± 17) |
| Appetite | 6 | 53 (± 28) | 63 (± 42) | Hallucination | 3 | 43 (± 12) |
| Decrease MS relapses | 4 | 58(± 10) | 70 (± 29) | Stomach pain | 3 | 20 (± 0) |
| Nausea | 3 | 80 (± 35) | 97 (± 6) | Headache | 2 | 40 (± 14) |
| Tremor | 2 | 35 (± 21) | 80 (± 14) | Chills | 1 | 50 (± 0) |
| Bowel/Bladder control | 1 | 50 (± 0) | 50 (± 0) | Memory problems | 1 | 70 (± 0) |
| Sexual functioning | 1 | 50 (± 0) | 80 (± 0) | Sweating | 1 | 30 (± 0) |
| Attention | 0 | Depression | 0 | |||
| Memory | 0 | Increase MS relapses | 0 | |||
| Vision | 0 | Shaking | 0 | |||
| Weight loss | 0 |
symptoms on quality of life (QoL) and effect of cannabis on symptom. Side-effects experienced as most
and second most bothersome/worrisome as well as perceived severity of side-effect on QoL. *Number of
respondents endorsing this option as either their most or second most response. Respondents could select
one option for their most and second most symptom/side-effect; if none was selected as their most option
they were not permitted to select a second most option. **All values are Mean (± SD) and associated with
symptoms and side-effects selected by All CUs. Perceived impact of symptom on QoL was measured
with a 10-point sliding scale from 0 (No impact) to 100 (Greatest impact possible); perceived effect of
cannabis on symptoms was measured with a 10-point sliding scale with 0 (Not at all) to 100 (Complete
relief and/or benefit). Perceived severity of side-effect on QoL was measured on a 10-point sliding scale
from 0 (Not severe) to 100 (Most severe).
Discussion
In this study, 38% of individuals endorsed currently using cannabis products for MS
symptoms. Although these results indicate a prevalence of use more than double the 16% of
current cannabis users reported in the 2017 North American Research Committee on Multiple
Sclerosis registry survey (Cofield et al., 2017), they are not surprising since the current study
occurred in a state where cannabis is available medically and recreationally. Self-reported
acceptance and willingness to use cannabis for MS is especially observed in areas where
cannabis is legal and available at least medically, if not also recreationally (Banwell et al., 2016;
Rudroff and Honce, 2017; Stuchiner et al., 2017; Brenton et al., 2018). These results also align
with other studies reporting increased prevalence of use among national and international
populations of PwMS (Linassi and Hader, 2003; Page et al., 2003; Chong et al., 2006; Banwell et
al., 2016; Rudroff and Honce, 2017; Stuchiner et al., 2017; Brenton et al., 2018; Rice and
Cameron, 2018). Thus, as the number of US states legalizing cannabis continues to grow,
clinicians are likely to continue to observe a growing interest in therapeutic cannabis use for
symptom relief among PwMS. Further along these lines, 74% of the current population reported
they would personally consider using cannabis for their MS, and 95% believed there should be
more research on Cannabis for MS, which is consistent with rates observed in prior studies (Page
et al., 2003; Rudroff and Honce, 2017; Brenton et al., 2018).
Other than the more general observation that use increases when cannabis is legalized,
few differences were observed between CUs and NUs that may explain why some PwMS choose
to use cannabis to manage MS symptoms. There were no sociodemographic differences between
NUs and CUs. Self-reported clinical characteristics (e.g., age of MS onset, symptom control, rate
of disability), as well as PRO data, were also similar between NUs and CUs. Only PDDS, which
is a measure of disability (Learmonth et al., 2013), was higher among CUs than NUs. While it
may be tempting to interpret this finding as being indicative of individuals seeking
complementary-alternative medicine (i.e., cannabis) when they are struggling with more severe
disease burden, alternative explanations exist, and further exploration is needed. However, along
these lines, it should be noted that 23% of CUs had never used prior to their diagnosis with MS
and 30% of CUs had only limited exposure to cannabis (i.e., had used cannabis less than 15
times in their lifetime). Thus, while 29% of CUs estimated that they had used cannabis 100 or
more times prior to their diagnosis with MS, individuals who it would seem were less likely to
start using cannabis regularly also began using later in life once they were diagnosed with MS.
Also, strictly medicinal users reported lower physical health and greater disability compared to
patients using for recreational purposes.
Concerning the symptoms for which PwMS are using cannabis to manage, this study’s
CUs reported using cannabis mostly for the treatment of pain, insomnia/poor sleep, and
spasticity/muscle tightness. Accordingly, a retrospective chart review of 4008 unique patients at
the RMMSC to identify the frequency with which five common MS symptom treatment
medications were prescribed revealed that 28% of patients had been prescribed Baclofen for pain
and muscle spasticity, 24% had been prescribed Gabapentin for pain and muscle spasticity, and
13% had been prescribed Dalfampridine for walking at some point (both past and active prescriptions were included).
Thus, cannabis is being used to manage symptoms for which other
prescription pharmaceutical drugs are available and with a higher rate of use (38%). Indeed,
other studies demonstrate that in some patients, cannabis use is perceived as less intoxicating and
resulted in reduced use of conventional pharmaceutical therapies (Page et al., 2003; Clark et al.,
2004; Lucas and Walsh, 2017), as common pharmaceutical therapies can have a range of adverse
effects ultimately resulting in limited benefit for patients (Beard et al., 2003; Fu et al., 2018).
The current data suggests that the side-effects of cannabis use are fairly-well tolerated by
PwMS. Seventy-nine percent of CUs reported that they experience no side-effects, which is
consistent with reports that many PwMS using cannabis appear to tolerate it well (Page et al.,
2003; Clark et al., 2004; Chong et al., 2006). However, it should be emphasized that self-report
data regarding side-effects may be of limited validity. For example, regarding cognition, it is
well-established that subjective cognitive report may be influenced by multiple factors (e.g.,
mood) and not necessarily predictive of objective test performance (Rohling et al., 2002; Farrin
et al., 2003). Furthermore, cannabis use, with cannabis likely representing a proxy for THC, has
been shown to impact aspects of cognition, especially among individuals with MS, though this
effect may be attenuated by CBD (Morgan et al., 2010; Schreiner and Dunn, 2012; Englund et
al., 2013; Romero et al., 2015). Clearly, further study is needed, especially as many individuals
seem to be trying to limit the side-effects/psychoactive effects of cannabis that they experience.
In the current study, this is demonstrated by the fact that 20% of CU’s preferred products that are
reported to contain only CBD and another 49% preferred products with similar quantities of
CBD and THC. Similarly, 85% of individuals who had only limited exposure to cannabis prior to
their diagnosis with MS preferred products with only CBD.
Regarding efficacy, CUs reported a 50% or greater relief /benefit for pain, insomnia, and
spasticity/muscle tightness. Also, interestingly, of the 35% of CUs who indicated they used it at
least once a week, 62% endorsed consuming cannabis like a prescription medication. It is
possible that the percentage of patients using cannabis like a prescription in the current study
reflects a tolerance for cannabis side-effects and experience that cannabis is more efficacious and
beneficial for symptom relief. In fact, one respondent even wrote that cannabis ―helps with pain
and sleep when pills don’t.‖ However, there was substantial variability in the amount and
cannabis products used across individuals to treat symptoms. For example, the proportion of CUs
reporting infrequent (i.e. 1-6 times/year or 2-3 times/month) versus frequent (once/week, 2-3
times/week, once a day, etc.) use was nearly evenly distributed at 43% and 57%, respectively.
Also, as indicated above, while 20% of CU’s preferred products that are reported to contain only
CBD and another 49% preferred products with similar quantities of CBD and THC, 9% preferred
THC products, and a review of the data indicates that CUs reported using cannabis to manage the
same symptoms regardless of their cannabinoid profile preference. Furthermore, PwMS who
consider themselves strictly medical users of cannabis tend to avoid combustion/smoking as a
method of consumption, but the consumption method overwhelmingly used by individuals who
do not consider their use strictly medicinal is combustion/smoking.
The above reported findings should be understood in the context of the limitations of this
study. The limitations of the current study largely revolve around the bias inherent to survey
data. For example, unfortunately, information was not systematically collected regarding
individuals who declined to participate. Thus, there may be some degree of selection bias in our
sample of PwMS, although, anecdotally, reasons given for declining survey participation ranged
from lack of time, to belief that cannabis use should not be legal, to fear of identification causing
legal/occupational difficulties. Also, regarding CUs more specifically, response bias may have
influenced their ratings of the impact that cannabis has on the management of their MS
symptoms, resulting in ratings that overestimate efficacy, and their report of side-effects,
resulting in underestimates of the undesirable effects of cannabis. Finally, there is concern that
the reported usage of THC and CBD products may be somewhat inaccurate. This concern has
less to do with response bias, though this cannot be excluded as a possibility, and more to do
with the findings of Vandrey et al. (2015) that many cannabis products were mislabeled
regarding the accuracy of THC and CBD content, though it should be noted that this study was
not performed in Colorado, where proficiency testing for medicinal cannabis flower was
established in 2016 and made mandatory for other cannabis products (e.g., edibles) in late 2017
by the state.
In summary, legalization efforts appear to be increasing the number of PwMS seeking out
cannabis as a complimentary-alternative medicine, with CUs self-reporting that their products of
choice are highly efficacious and noting minimal side-effects. In addition to changing attitudes
towards cannabis use, legalization has also resulted in a flood of available products with
differing levels of cannabinoids and methods of ingestion. While this has resulted in some good,
such as the fact that PwMS who consider themselves strictly medical users tend to avoid
combustion/smoking as a method of consumption, it also makes generalizing specific research
findings regarding cannabis to specific instances/types of use rather difficult. In order to provide
sound evidence-based advice to patients, further objective studies regrading both treatment
effects and unwanted side-effects of varying levels of particular cannabinoids, including but not
limited to THC and CBD, are needed, as is further objective evidence on the health effects of
differing methods of ingestion (e.g., tincture versus vaping versus combustion/smoking). Fortunately, 87% of CUs in the current study reported comfortable discussing their use with their physicians.
Acknowledgements
We thank the Rocky Mountain Multiple Sclerosis Center for their support.
Funding
This work was supported by the Rocky Mountain Multiple Sclerosis Center.
References
Andre CM, Hausman JF, Guerriero G. 2016. Cannabis sativa: The Plant of the Thousand and
One Molecules. Front Plant Sci 7:19.
Atakan Z. 2012. Cannabis, a complex plant: different compounds and different effects on
individuals. Therapeutic Advances in Psychopharmacology 2:241-254.
Banwell E, Pavisian B, Lee L, Feinstein A. 2016. Attitudes to cannabis and patterns of use
among Canadians with multiple sclerosis. Mult Scler Relat Disord 10:123-126.
Beard S, Hunn A, Wight J. 2003. Treatments for spasticity and pain in multiple sclerosis: a
systematic review. Health Technol Assess 7:iii, ix-x, 1-111.
Belendiuk KA, Baldini LL, Bonn-Miller MO. 2015. Narrative review of the safety and efficacy
of marijuana for the treatment of commonly state-approved medical and psychiatric
disorders. Addict Sci Clin Pract 10:10.
Boggs, DL, Nguyen, JD, Morgenson, D., Taffe, MA, Ranganathan, M. (2018). Clinical and
preclinical evidence for functional interactions of cannabidiol and Δ9-
tetrahydrocannabinol. Neuropsychopharmacology, 43: 142-154.
Booz, GW. (2011). Cannabidiol as an emergent therapeutic strategy for lessening the impact of
inflammation on oxidative stress. Free Radical Bilogy & Medicine. 51: 1054-1061.
Brenton JN, Schreiner T, Karoscik K, Richter M, Ferrante S, Waldman A, Banwell B. 2018.
Attitudes, perceptions, and use of marijuana in youth with multiple sclerosis. J Neurol
265:417-423.
Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. 2006. Cannabis use in patients
with multiple sclerosis. Mult Scler 12:646-651.
Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. 2004. Patterns of cannabis use among
patients with multiple sclerosis. Neurology 62:2098-2100.
Cofield SS, Salter A, Tyry T, Crowe C, Cutter GR, Fox RJ, Marrie RA. 2017. Perspectives on
marijuana use and effectiveness: A survey of NARCOMS participants. Neurol Clin Pract
7:333-343.
Consroe P, Musty R, Rein J, Tillery W, Pertwee R. 1997. The perceived effects of smoked
cannabis on patients with multiple sclerosis. Eur Neurol 38:44-48.
Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, Gouaux B. 2012. Smoked
cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. Cmaj
184:1143-1150.
Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg
A, Brenneisen R, Holt D, Feilding A, Walker L, Murray RM, Kapur S. 2013. Cannabidiol
inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory
impairment. J Psychopharmacol 27:19-27.
Farrin L, Hull L, Unwin C, Wykes T, David A. 2003. Effects of depressed mood on objective
and subjective measures of attention. J Neuropsychiatry Clin Neurosci 15:98-104.
Fife TD, Moawad H, Moschonas C, Shepard K, Hammond N. 2015. Clinical perspectives on
medical marijuana (cannabis) for neurologic disorders. Neurol Clin Pract 5:344-351.
Fu X, Wang Y, Wang C, Wu H, Li J, Li M, Ma Q, Yang W. 2018. A mixed treatment
comparison on efficacy and safety of treatments for spasticity caused by multiple
sclerosis: a systematic review and network meta-analysis. Clin Rehabil 32:713-721.
Giacoppo S, Mandolino G, Galuppo M, Bramanti P, Mazzon E. 2014. Cannabinoids: new
promising agents in the treatment of neurological diseases. Molecules 19:18781-18816.
Gupta S, Fellows K, Weinstock-Guttman B, Hagemeier J, R. Z, Ramanathan M. 2018. Marijuana
Usage by Multiple Sclerosis Patients. International Journal of MS Care. Advance online
publication. http://ijmsc.org/doi/pdf/10.7224/1537-2073.2017-112
Hays, R. D., Bjorner, J. B., Revicki, D. A., Spritzer, K. L., & Cella, D. 2009. Development of
physical and mental health summary scores from the patient-reported outcomes
measurement information system (PROMIS) global items. Quality of Life Research,
18(7), 873-880.
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D. 2014. Systematic
review: efficacy and safety of medical marijuana in selected neurologic disorders: report
of the Guideline Development Subcommittee of the American Academy of Neurology.
Neurology 82:1556-1563.
Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. 2013. Validation of patient
determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC
Neurol 13:37.
Linassi GA, Hader WJ. 2003. Perceived Effects of Marijuana Use By MS Patients in
Saskatchewan—A Pilot Study. International Journal of MS Care 5:139-150.
Lucas P, Walsh Z. 2017. Medical cannabis access, use, and substitution for prescription opioids
and other substances: A survey of authorized medical cannabis patients. Int J Drug Policy
42:30-35.
Morgan CJ, Schafer G, Freeman TP, Curran HV. 2010. Impact of cannabidiol on the acute
memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic
study [corrected]. Br J Psychiatry 197:285-290.
National Institute of Neurological Disorders and Stroke (NINDS user manual for the Quality of
Life in Neurological Disorders (Neuro-QoL) measures, Volume 2.0. Marchhttp://www.healthmeasures.net/images/neuro_qol/Neuro_QOL_Scoring_Manual_
Mar2015.pdf Accessed April 2018 In.
Page SA, Verhoef MJ, Stebbins RA, Metz LM, Levy JC. 2003. Cannabis use as described by
people with multiple sclerosis. Can J Neurol Sci 30:201-205.
Rice J, Cameron M. 2018. Cannabinoids for Treatment of MS Symptoms: State of the Evidence.
Curr Neurol Neurosci Rep 18:50.
Rohling ML, Green P, Allen LM, 3rd, Iverson GL. 2002. Depressive symptoms and
neurocognitive test scores in patients passing symptom validity tests. Arch Clin
Neuropsychol 17:205-222.
Romero K, Pavisian B, Staines WR, Feinstein A. 2015. Multiple sclerosis, cannabis, and
cognition: A structural MRI study. Neuroimage Clin 8:140-147.
Rudroff T, Honce JM. 2017. Cannabis and Multiple Sclerosis-The Way Forward. Front Neurol 8:299.
Schreiner AM, Dunn ME. 2012. Residual effects of cannabis use on neurocognitive performance
after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol 20:420-429.
Stuchiner T, Baraban E, Chen C, Cohan S. 2017. Use of Medical Marijuana for the Relief of
Multiple Sclerosis (MS) Symptoms in a Community Cohort: Survey from the Pacific
Northwest MS Registry (P3.356). Neurology 88.
Svendsen KB, Jensen TS, Bach FW. 2004. Does the cannabinoid dronabinol reduce central pain
in multiple sclerosis? Randomised double blind placebo controlled crossover trial. Bmj
329:253.
Thompson, AJ, Banwell, BL, Barkhof, F, et al. (2018). Diagnosis of multiple sclerosis: 2017
revisions of the McDonald Criteria. Lancet Neurol 17: 162–73.
Vandrey, R, Raber, JC, Raber, M. Douglass, B, Miller, C, Bonn-Miller, MO. (2015),
Cannabinoid Dose and Label Accuracy in Edible Medical Cannabis Products. Journal of
the American Medical Association; 313: 2491.
Wade DT, Makela P, Robson P, House H, Bateman C. 2004. Do cannabis-based medicinal
extracts have general or specific effects on symptoms in multiple sclerosis? A double-
blind, randomized, placebo-controlled study on 160 patients. Mult Scler 10:434-441.
Wade DT, Makela PM, House H, Bateman C, Robson P. 2006. Long-term use of a cannabis-
based medicine in the treatment of spasticity and other symptoms in multiple sclerosis.
Mult Scler 12:639-645.
Zajicek JP, Apostu VI. 2011. Role of cannabinoids in multiple sclerosis. CNS Drugs 25:187-201.
Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, Nunn AJ, Teare LJ,
Fox PJ, Thompson AJ. 2005. Cannabinoids in multiple sclerosis (CAMS) study: safety
and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 76:1664-1669.
This originally appeared at https://www.msard-journal.com/article/S2211-0348(18)30515-7/fulltext