This originally appeared at https://pubmed.ncbi.nlm.nih.gov/25796592/
Abstract
An updated systematic review of randomized con-
trolled trials examining cannabinoids in the treatment of
chronic non-cancer pain was conducted according to PRIS
MA guidelines for systematic reviews reporting on health care
outcomes. Eleven trials published since our last review met
inclusion criteria. The quality of the trials was excellent. Sev-
en of the trials demonstrated a significant analgesic effect.
Several trials also demonstrated improvement in secondary
outcomes (e.g., sleep, muscle stiffness and spasticity). Ad-
verse effects most frequently reported such as fatigue and
dizziness were mild to moderate in severity and generally well
tolerated. This review adds further support that currently
available cannabinoids are safe, modestly effective analgesics
that provide a reasonable therapeutic option in the manage-
ment of chronic non-cancer pain.
Introduction
Chronic pain is a growing public health problem affecting
approximately one in five people and predicted to in-
crease to one in three over the next two decades (Blyth
et al. 2001; Moulin et al. 2002; Breivik et al. 2006). The
prevalence of chronic pain is likely to increase as the
population ages and as medical advances continue to im-
prove survival related to cancer, serious injury and dis-
eases that previously would have been fatal, such as
HIV, but have left the survivors with serious neuropathic
pain conditions (Lynch 2011). Currently available agents
(eg. antidepressant and anticonvulsant analgesics, opioids
and nonsteroidal anti-inflammatory drugs) (Finnerup et al.
2010) are inadequate to control all pain or are associated
with limiting side effects (eg. most problematic being se-
dation with the antidepressant and anticonvulsant group,
constipation with the opioids and gastrointestinal and car-
diovascular effects with the NSAIDs) (Lynch 2008).
There is a critical need for new treatments.
In this context, many people with chronic pain are
turning to other therapies including cannabinoids (Ware
et al. 2003). Due to patient demand, several nations (or
states within countries) have developed programs to allow
people with serious health conditions to access cannabis
(marijuana) for medicinal purposes. Most of these pro-
grams (e.g., Canada, Israel, Netherlands, several US
States) require physician or nurse practitioner support
for the individual patient to be approved for access. Med-
ical professionals have called for more research regarding
both potential therapeutic and adverse effects of cannabi-
noids (Kahan et al. 2014). This is an updated systematic
review of controlled trials done since the previous system-
atic review regarding cannabinoids in the treatment of
chronic non-cancer pain (Lynch and Campbell 2011).
Methods
We conducted a systematic review following PRISMA guide-
lines (Liberati et al. 2009). Initially a literature search was
undertaken to retrieve Randomized Control Trials (RCT) on
the efficacy of cannabinoids in the treatment for chronic pain.
The databases searched were: PubMed, Embase, CINAHL
(EBSCO), PsycInfo (EBSCO), The Cochrane Library (Wi-
ley), ISI Web of Science, ABI Inform (Proquest), Dissertation
Abstracts (Proquest), Academic Search Premier (EBSCO),
Clinical Trials.gov, TrialsCentral.org, individual pharmaceuti-
cal company trials sites for Eli Lilly and GlaxoSmithKline,
OAIster (OCLC), LILACS, and Google Scholar. The searches
were updated from the date of last search in 2010 to October
2014 and were not limited by language. The search retrieved
all articles assigned the Medical Subject Headings (MeSH)
Cannabis, Cannabinoids, Cannabidiol (CBD), Marijuana
Smoking and delta-9-Tetrahydrocannabinol (THC) as well as
those assigned the Substance Name tetrahydrocannabinol-
cannabidiol combination. Cannabidiol (CBD) is the main
n o n p s y c h o t r o p i c p h y t o c a n n a b i n o i d a n d de l t a – 9 –
tetrahydrocannabinol (THC) the main psychoactive cannabi-
noid in the cannabis plant (Skaper and DiMarzo 2012). To this
set was added those articles containing any of the keywords
cannabis, cannabinoid*, marijuana, marihuana, dronabinol
or tetrahydrocannabinol. Members of this set containing the
MeSH heading Pain or the keyword Bpain^ were passed
through the BClinical Queries: therapy/narrow^ filter to arrive
at the final results set of RCTs. The search strategy was
adapted for and run in the other databases by an experienced
medical librarian.
Inclusion and Exclusion Criteria
Included in this review were RCTs comparing a cannabinoid
with a placebo or active control group where pain was a re-
ported measured outcome in subjects with chronic non-cancer
pain. Relevant outcomes included any scale measuring pain.
This might include a numeric rating scale (NRS), a visual
analog scale (VAS), the Neuropathic Pain Scale (NPS) or the
McGill Pain Questionnaire. Excluded were trials where pain
was not reported as an outcome, trials regarding experimental
or acute pain and cancer pain, preclinical studies, abstracts,
letters and posters where the full study was not published.
Data Extraction and Validity Scoring
Both authors independently read the included articles and
completed the assessment of methodological validity using
the modified seven point, four item Oxford scale (Fig. 1).
Discrepancies on the validity assessment were resolved through discussion. Trials that did not include randomization
were not included and a score of 1 or more on this item of the
Oxford Scale was required.
Data extracted included information about the specific pop-
ulation studied, number of subjects randomized and complet-
ed, outcomes, summary measures, trial duration, results and
adverse events. Information about the most frequently report-
ed or serious adverse events was extracted.

Results
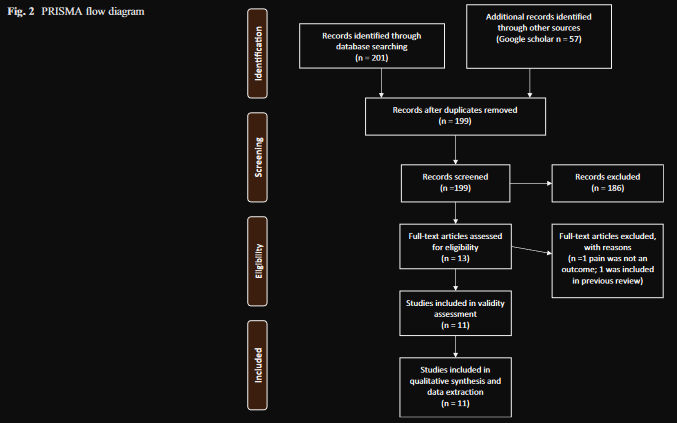
Trial Flow
A total of 201 citations were initially identified through data-
base searches. Duplicates were removed and unique citations
identified through Google Scholar were added, resulting in a
total of 199 citations. Research assistants and the review team
screened the studies for content relevance and study design at
the title and abstract level to exclude 186 results, leaving 13
trials to be screened by examining the full text. One study was
excluded after reading the full text as it had not included pain
as an outcome, and one was removed as it had been included
in the previous review, leaving 11 studies that received quality
screening and underwent data extraction (Fig. 2).
Primary Outcome-Efficacy
There were 11 randomized controlled trials published from
2010 to 2014 involving 1185 subjects that met inclusion criteria.
The quality of trials was excellent with a mean score
of 7 (range 5–7) on the Modified Oxford Scale. In 7 studies
pain was the primary outcome, while in 4 studies other out-
comes were stated as primary outcome (sleep, headache fre-
quency, spasticity) with pain as a secondary outcome. Overall
7 studies demonstrated that the cannabinoid under study ex-
hibited an analgesic effect that was significantly better than the
control. In 9 studies the control was a placebo, while in 2
studies the cannabinoid was compared to an active control
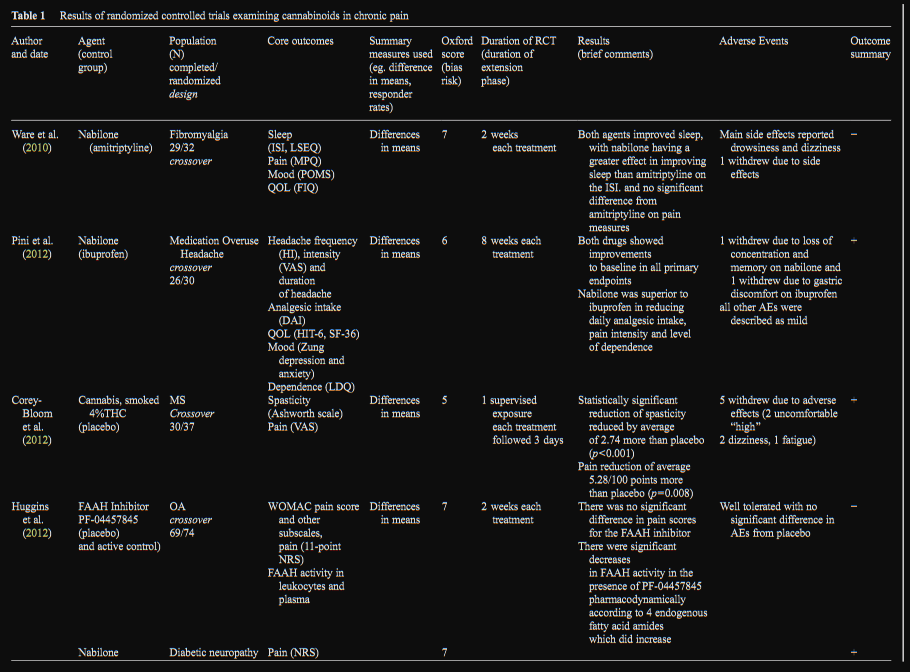
(nabilone and ibuprofen) (Table 1).
Nabilone
Nabilone is a synthetic analog of THC approved by the FDA
over 25 years ago for treatment of chemotherapy induced
nausea and vomiting (Pertwee 2012). Four studies examined
the efficacy of nabilone. In a study of medication overuse
headache (N=26/30), nabilone was superior to ibuprofen in
reducing daily analgesic intake, pain intensity and level of
dependence and was equally efficacious in reducing frequen-
cy (Pini et al. 2012). In a study of patients with painful diabetic
neuropathy (N=25/26) nabilone was significantly more effec-
tive than placebo in reducing pain with significant improve-
ments in secondary measures of anxiety and sleep (Toth et al.
2012). In a study using amitriptyline as an active control examining sleep with pain as a secondary measure in fibro-
myalgia (N = 29/32), both agents improved sleep with
nabilone demonstrating significantly more improvement in
sleep quality on one of the sleep measures; there was no sig-
nificant impact on pain, however subjects started with a rela-
tively low mean pain score of 2.3/10 (Ware et al. 2010). In a
study of MS pain (N=14/15) nabilone was demonstrated to
improve pain significantly more than placebo in combination
with gabapentin according to both the VAS and patient global
assessment of change (Turcotte et al. 2014).
Oral Mucosal Cannabis Spray and Oral Cannabis
Extract
Three RCTs examined an oromucosal cannabis spray (each
spray delivers 2.7 mg of THC and 2.5 mg of CBD) and 1
examined an oral cannabis extract (doses 5–25 mg daily with
CBD between 29 and 72 % that of THC). In neuropathic pain
associated with allodynia (N=173/246), the oral mucosal can-
nabis spray demonstrated a significant analgesic effect with
improvements in sleep and subject global impression of
change (Serpell et al. 2014). In a study involving neuropathic
pain in MS (N=297/339) oral mucosal cannabis spray dem-
onstrated a reduction in pain compared to placebo at 10 weeks;
however at 14 weeks, pain scores did not differ between the oral mucosal cannabis spray and placebo groups
(Langford et al. 2013). In a pilot study of chemotherapy
induced neuropathic pain (N=16/18), NRS pain scores
did not demonstrate a statistically significant difference
but a responder analysis found 5 of the 16 completers
reported a 2 point or greater reduction with an overall
NNT=5. Given that more than a quarter of patients with
this highly intractable type of pain responded to oral
mucosal cannabis spray over placebo, the authors con-
cluded further study was warranted (Lynch et al. 2014).
One study examined an oral cannabis extract in multiple
sclerosis with spasticity as the primary measure and
pain as a secondary measure (N =224/259) and found
relief from muscle stiffness pain and sleep was twice
as good with the cannabis extract than with placebo
(Zajicek et al. 2012).
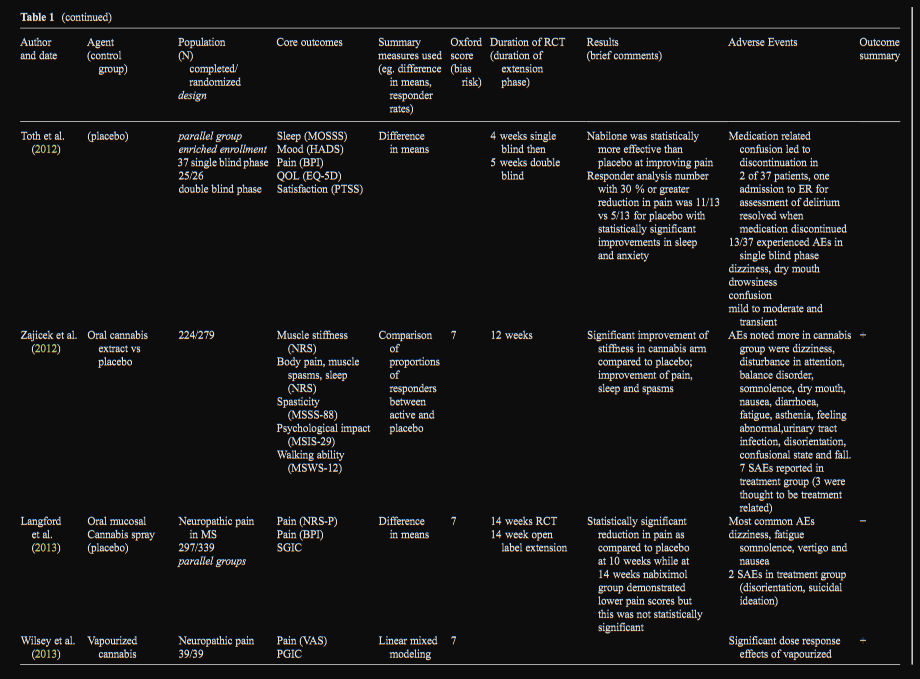
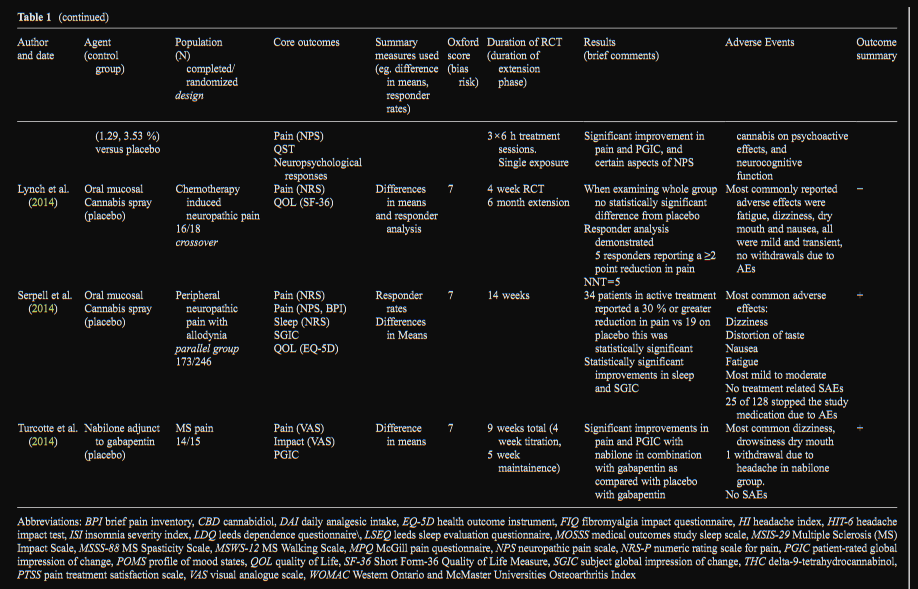
Cannabis (Smoked or Vaporized)
Since the 2010 systematic review, there have been 2 further
controlled trials examining smoked (1 trial) or vaporized (1
trial) cannabis. In neuropathic pain, cannabis containing both
a lower dose (1.29 % THC) and higher dose (3.53 % THC)
delivered by vaporizer demonstrated a significant analgesic
response as compared to placebo with an NNT for 30 % re-
duction of 3.2 and 2.9 respectively for low and higher dose
cannabis (Wilsey et al. 2013). In a study of MS spasticity and
pain, smoked cannabis containing 4 % THC demonstrated a
significant antispasticity and analgesic effect compared with
placebo (Corey-Bloom et al. 2012).
FAAH Inhibitor
There was one study examining a novel fatty acid amide
hydrolase inhibitor (FAAH). FAAH is one of the main
enzymes known to break down the endogenous cannabi-
noid, anandamide, as well as noncannabinoid fatty acid
amides (Cravatt et al. 2001). FAAH inhibition has been
found to elicit antinociceptive effects in animal models of
arthritis (Schuelert et al. 2011). In spite of promising pre-
clinical data, the single clinical trial examining a FAAH
inhibitor (PF-04457845) did not find a significant im-
provement in pain associated with osteoarthritis of the
knee (N=69/74). Interestingly there was a significant in-
crease in 4 endogenous fatty acids [anandamide (AEA),
oleoylethanolamide (OEA), palmitoylethanolamide
(PEA), and linoleoylethanolamide (LEA)], which demon-
strated a biological effect, but was not associated with a
reduction of pain (Huggins et al. 2012).
Adverse Events
All studies included specific information on adverse effects;
detailed findings are presented in Table 1. The adverse effects
seen with all cannabinoids were similar with drowsiness or
fatigue reported most frequently and dizziness, dry mouth,
nausea and cognitive effects reported in most trials. In the vast
majority the adverse effects were mild to moderate in severity,
transient and well tolerated. Regarding serious adverse events
(SAEs); in a study of oral cannabis extract in 279 patients with
MS there were 3 adverse events described as serious and med-
ication related, these included urinary tract infection, head
injury and interstitial lung disease (Zajicek et al. 2012). In
a study examining nabilone in treatment of 37 patients with
diabetic neuropathy one patient was seen in the emergency
room for assessment of delirium which resolved when the
medication was discontinued (Toth et al. 2012) and in a study
of the oral mucosal cannabis spray in 339 patients with neuropathic pain associated with MS there were 2 SAEs in the treatment group (suicidal ideation and disorientation)
(Langford et al. 2013).
Discussion
Efficacy and Harm
This is an update to a previous systematic review examining
RCTs using cannabinoids for treatment of chronic non-cancer
pain. The current review found 11 RCTs published since our
last review, 7 of which demonstrated significant analgesic
effects by the cannabinoids studied. Several trials also report-
ed benefits in sleep and 2 of the MS trials also demonstrated
benefits in muscle stiffness and spasticity. Drug related ad-
verse effects consisted primarily of fatigue, dizziness, dry
mouth, nausea and disturbances in cognition and were mild
to moderate, transient and generally well tolerated. The find-
ings of the current review extend and are consistent with those
from the previous review (Lynch and Campbell 2011) such
that combined there are a total of 22 of 29 RCTs demonstrating that cannabinoids demonstrate a modest analgesic effect and are safe in the management of chronic pain.
Limitations
The main limitations to these findings are that most of the
trials were of short duration, with relatively small sample sizes
and modest effect sizes. There is a need for larger and longer
trials to confirm efficacy signals shown by the smaller ‘proof
of concept’ studies, and for longer term monitoring of patients
using cannabinoids for long term safety considerations. Cur-
recently available cannabinoids only appear to reduce pain to a modest degree, similar to all medications currently available for the treatment of chronic pain.
One message from this review is that it is perhaps ill-
advised to treat all cannabinoids the same; talk of Bmedical
marijuana^ often lumps all these compounds and formulations
together. In fact there are very important pharmacokinetic dif-
ferences between modes of delivery (e.g., oral versus inhaled),
differences in cannabinoid profiles (e.g., the presence of CBD
in different amounts), and source of cannabinoid (plant based
complex botanicals versus synthetic single molecules). Such
diversity of approach is welcomed as each study adds value to
the overall weight of evidence that cannabinoids as a drug
class have analgesic potential, but distinctions must be made
when citing these studies to ensure that the conclusions are not
drawn more widely than is justified.
The decision as to whether the degree of pain relief obtain-
ed from using cannabinoids is clinically meaningful will re-
main a decision based on informed decision making between
the patient and their health care provider; however we feel that
cannabinoids have demonstrated sufficient analgesic potential
to be included in serious discussions around therapeutic op-
tions in the treatment of chronic pain.
Conclusions
In summary the current systematic review provides further
support that cannabinoids are safe, demonstrate a modest an-
algesic effect and provide a reasonable treatment option for
treatment chronic non-cancer pain.
Acknowledgments The authors thank Robin Parker for her excel-
lent assistance in conducting the literature search for this review
Conflict of Interest ML is a founding director of Panag Pharm Inc
and is medical advisor to Abide Therapeutics both start up companies
focused on development of nonpsychotropic cannabinoids for treatment
of pain and other health conditions, she also sits on the Board of the
Canadian Consortium for the Investigation of Cannabinoids (CCIC) a
nonprofit organization dedicated to research and education on
cannabinoids.
MW has received a grant from Prairie Plant Systems for a clinical trial
of cannabis for pain management. MW is Executive Director of the Ca-
nadian Consortium for the Investigation of Cannabinoids (CCIC), a non-
profit organization dedicated to research and education on cannabinoids.
References
Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ
(2001) Chronic pain in Australia: a prevalence study. Pain 89:127–
134
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D (2006) Survey
of chronic pain in Europe: prevalence, impact on daily life and
treatment. Eur J Pain 10:287–333
Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley BA,
Gouaux B (2012) Smoked cannabis for spasticity in multiple scle-
rosis: a randomized, placebo-controlled trial. CMAJ 184:1143–1150
Cravatt BF, Demarest K, Patricelli PM, Bracey MH, Giang DK, Martin
BR, Lichtman AH (2001) Supersensitivity to anandamide and en-
hanced endogenous cannabinoid signaling in mice lacking fatty
acide amide hydrolase. Proc Natl Acad Sci 98:9371–9376
Finnerup NB, Sindrup SH, Jensen TS (2010) The evidence for pharma-
cological treatment of neuropathic pain. Pain 150:573–581
Huggins JP, Smart TS, Langman S, Taylor L, Young T (2012) An effi-
cient randomised, placebo-controlled clinical trial with the irrevers-
ible fatty acid amide hydrolase-1 inhibitor PF-04457845, which
modulates endocannabinoids but fails to induce effective analgesia
in patients with pain due to osteoarthritis of the knee. Pain 153:
1837–1846
Kahan M, Srivastava A, Spithoff S, Bromley L (2014) Prescribing
smoked cannabis for chronic noncancer pain: preliminary recom-
mendations. Can Fam Physician 60:1083–1090
Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W,
Ratcliffe S (2013) A double-blind, randomised, placebo-controlled,
parallel-group study of THC? CBD oralmucosal spray in combina-
tion with the existing treatment regimen, in the relief of central
neuropathic pain in patients with multiple sclerosis. J Neurol 260:
984–997
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ionnidis
JPA, CLarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The
PRISMA statement for reporting systematic reviews and meta-
analysis of studies that evaluate health care interventions: explana-
tion and elaboration. Ann Int Med 15:W-65–W-94
Lynch ME (2008) The pharmacotherapy of chronic pain. Rheum Dis Clin
N Am 34:369–385
Lynch ME (2011) The need for a Canadian pain strategy. Pain Res Manag
16:77–80
Lynch ME, Campbell F (2011) Cannabinoids for treatment of chronic
non-cancer pain; a systematic review of randomized controlled tri-
als. Br J Clin Pharmacol 72:735–744
Lynch ME, Cesar-Rittenberg P, Hohmann AG (2014) A double-blind,
placebo-controlled, corssover pilot trial with extension using oral
mucosal cannabinoid extract for treatment of chemotherapy-
induced neuropathic pain. J Pain Symptom Manag 47:166–
17323742737
Moulin D, Clark AJ, Speechly M, Morley-Forster P (2002) Chronic pain
in Canada, prevalence, treatment, impact and the role of opioid
analgesia. Pain Res Manag 7:179–184
Pertwee RG (2012) Targeting the endocannabinoid system with cannabi-
noid receptor agonists: pharmacological strategies and therapeutic
possibilities. Philos Trans R Soc Lond B Biol Sci 367:3353–3363
Pini LA, Guerzoni S, Cainazzo MM, Ferrari A, Sarchielli P, Tiraferri I,
Ciccarese M, Zappaterra M (2012) Nabilone for the treatment of
medication overuse headache: results of a preliminary double-blind,
active-controlled, randomized trial. J Headache Pain 13:677–684
Schuelert N, Johnson MP, Oskins JL, Jassal K, Chambers MG,
McDougall JJ (2011) Local application of the endocannabinoid hy-
drolysis inhibotor URB597 reduces nociception in spontaneous and
chemically induced models of osteoarthritis. Pain 152:975–981
Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, Ehler
E (2014) A double-blind, randomized, placebo-controlled, parallel
group study of THC/CBD spray in peripheral neuropathic pain treat-
ment. Eur J Pain 18:999–1012
Skaper SD, DiMarzo V (2012) Endocannabinoids in nervous system
health and disease: the big picture in a nutshell. Phil Trans R Soc
B 367:3193–3200
Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, Garven A (2012)
An enriched-enrolment, randomized withdrawal, flexible-dose, dou-
ble-blind, placebo controlled, parallel assignment efficacy study of
nabilone as adjuvant in the treatment of diabetic peripheral neuro-
pathic pain. Pain 153:2073–2082
Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F (2014)
Nabilone as an adjunctive to gabapentin for multiple sclerosis-
induced neuropathic pain: a randomized controlled trial. Pain Med.
doi:10.1111/pme.12569
Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ (2003) Cannabis
use for chronic non-cancer pain: results of a prospective survey. Pain
102(1–2):211–216
Ware MA, FItzcharles M, Lawrence J, Shir Y (2010) The effects of
nabilone on sleep in fibromyalgia: results of a randomized con-
trolled trial. Anesth Analg 110:604–610
Wilsey B, Marcotte TD, Deutsch R, Gouaux B, Sakai S (2013) Low-dose
vaporised cannabis significantly improves neuropathic pain. J Pain
14:136–148
Zajicek JP, Hobart JC, Slade A, Barnes D, M. PG (2012) Multiple scle-
rosis and extract of cannabis: results of the MUSEC trial. J Neurol
Neurosurg Psychiatry 83:1125–1132