- PMID: 26187456
- PMCID: PMC4536087
- DOI: 10.1016/j.drugalcdep.2015.05.013
Abstract
Background: Evidence suggests that the cannabinoid system is involved in the maintenance of opioid dependence. We examined whether dronabinol, a cannabinoid receptor type 1 partial agonist, reduces opioid withdrawal and increases retention in treatment with extended release naltrexone (XR-naltrexone).
Methods: Opioid dependent participants were randomized to receive dronabinol 30mg/d (n=40) or placebo (n=20), under double-blind conditions, while they underwent inpatient detoxification and naltrexone induction. Before discharge all participants received an injection of XR-naltrexone, with an additional dose given four weeks later. Dronabinol or placebo was given while inpatient and for 5 weeks afterwards. The primary outcomes were the severity of opioid withdrawal, measured with the Subjective Opioid Withdrawal Scale, and retention in treatment at the end of the inpatient phase and at the end of the 8-week trial.
Results: The severity of opioid withdrawal during inpatient phase was lower in the dronabinol group relative to placebo group (p=0.006). Rates of successful induction onto XR-naltrexone (dronabinol 66%, placebo 55%) and completion of treatment (dronabinol 35%, placebo 35%) were not significantly different. Post hoc analysis showed that the 32% of participants who smoked marijuana regularly during the outpatient phase had significantly lower ratings of insomnia and anxiety and were more likely to complete the 8-week trial.
Conclusion: Dronabinol reduced the severity of opiate withdrawal during acute detoxification but had no effect on rates of XR-naltrexone treatment induction and retention. Participants who elected to smoke marijuana during the trial were more likely to complete treatment regardless of treatment group assignment.
INTRODUCTION
Rates of prescription opioid and heroin use and related morbidity and mortality continue to grow at an alarming rate (SAMHSA, 2013) with a parallel increase in unintentional overdose deaths (CDC, 2012). Treatment with opioid agonists, methadone and buprenorphine is a time-honored and effective approach to manage opioid dependence, however, agonists are not effective for all patients. Approximately 50% of individuals continue using opioids or other drugs, or drop out during the first 6 months of treatment (Mattick et al., 2008; Soyka et al., 2008). Treatment with the opioid receptor antagonist is an alternative treatment approach that has the potential to address some of the limitations of agonists and attract and retain more patients in stable long-term recovery (SAMHSA, 2012). In patients who are able to initiate treatment with extended-release naltrexone, the overall effectiveness of treatment is comparable with agonists with regards to treatment retention (50–70%) with lower rates of ongoing opioid use (Bisaga et al., 2014; Brooks et al., 2010; Comer et al., 2006; Krupitsky et al., 2011).
Initiation of naltrexone treatment is best accomplished while the patient is completing residential treatment. For patients who are opioid dependent, initiation of naltrexone during detoxification is associated with significant withdrawal symptoms. An alternative approach, to wait for 7–10 days post-detoxification before administering naltrexone, results in high rates of relapse. Strategies for easing the rapid transition from agonist to antagonist generally involve a brief course of buprenorphine, followed by use of non-opioid medications to attenuate withdrawal symptoms (Sigmon et al., 2012). Nonetheless withdrawal symptoms can still be substantial, reducing success rates of naltrexone induction. Further, patients who start naltrexone frequently experience protracted withdrawal symptoms that persist for 2–3 weeks and may further limit naltrexone’s acceptability and adherence. Approximately 30–40% of individuals who start detoxification leave treatment prior to receiving the first injection of XR-naltrexone and another 30–40% will drop out during the first 2 months of outpatient treatment (Bisaga et al., 2014; Comer et al., 2006; Nunes et al., 2006).
Ascertaining an adjunctive medication to alleviate acute and protracted withdrawal symptoms could have a significant impact on improving effectiveness of naltrexone and help with its widespread implementation. Observational data from several independent studies, and clinical experience, suggest that patients who use marijuana following induction onto naltrexone have better retention in treatment as compared to individuals who do not use marijuana (Church et al., 2001; Raby et al., 2009). This finding suggests marijuana may help alleviate withdrawal symptoms early in the course of naltrexone treatment and points to the role of the endocannabinoid system in preventing opioid dependence relapse.
The endocannabinoid system is involved in the maintenance of drug addiction, and targeting this system has been proposed as an approach to treatment (Panlilio et al., 2013; Scavone et al., 2013a; Serrano and Parsons, 2011). The cross-regulation between cannabinoid and opioidergic pathways has been well documented in preclinical studies (Robledo et al., 2008). Chronic exposure to opioids produces profound changes in the endocannabinoid system (Lopez-Moreno et al., 2008; Parolaro et al., 2010), possibly contributing to behavioral abnormalities emerging during early abstinence. Preclinical studies show that cannabinoid agonists reduce the severity of precipitated opioid withdrawal (Frederickson et al., 1976; Lichtman et al., 2001; Vela et al., 1995; Yamaguchi et al., 2001) possibly by modulating opioid signaling in noradrenergic cells of coeruleo-cortical pathways (Scavone et al., 2013a). Therefore, targeting cannabinoid systems may be a viable therapeutic strategy in opioid dependence.
We conducted a double blind, placebo-controlled trial of dronabinol in combination with XR-naltrexone among opioid-dependent patients. Dronabinol is oral synthetic Δ9tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana and a cannabinoid receptor type 1 partial agonist (Pertwee, 2009). We hypothesized that administering dronabinol during detoxification and the first weeks of treatment with XR-naltrexone would diminish the severity of opioid withdrawal and, as a result, improve treatment retention and reduce rates of opioid use as compared to treatment with placebo.
METHODS
Participants
Opioid-dependent individuals seeking treatment were evaluated at an outpatient research clinic using the Structured Clinical Interview for DSM-IV (First et al., 1995) and a clinical interview assessing substance abuse severity. Medical evaluation included history, laboratory tests, electrocardiogram together with physical and psychiatric exam. The Institutional Review Board of the New York State Psychiatric Institute approved the study.
Eligible individuals were between 18–60 years old who met criteria for current opioid dependence and were able to give an informed consent to participate. Individuals with unstable medical or psychiatric disorders were excluded. Other exclusion criteria included: 1) physiological dependence on alcohol or sedative-hypnotics; 2) history of recent opioid overdose; 3) treatment with opioids for chronic pain or regular use of methadone; and 4) treatment with psychotropic medications.
We only enrolled participants who had experience smoking marijuana, to avoid exposing participants naïve to THC effects. We excluded participants who were at risk for marijuana withdrawal during inpatient treatment (i.e., those smoking multiple times every day), and excluded participants with cannabis dependence in remission to minimize the risk of relapse.
We evaluated 517 individuals; of whom 170 declined to participate and 190 were not eligible to participate (84 had significant medical problems, 46 had significant psychiatric co-morbidities, 14 were taking other psychotropic medications, and 46 were not eligible for other reasons; see Figure 1). In addition, 97 participants entered other naltrexone-based treatment studies conducted concurrently at the clinic. A total of 60 individuals provided informed consent and entered the study. Participants were stratified on baseline opioid use (high/low) and age (younger/older). Low use group included participants using five or fewer bags/d (200 mg of morphine equivalent or less) versus six or more bags/d for high use group. Younger group included participants 39 years old and younger versus 40 years and older in older group. There were 22 participants in older/high use stratum, 6 in older/low use stratum, 21 in younger/high use stratum and 11 in younger/low use stratum. Within each stratum participants were randomized to dronabinol 30 mg (n=40) or placebo (n=20) with uneven randomization to obtain additional clinical experience (dosing, safety) with dronabinol.
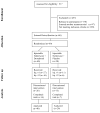
Study Procedures
Enrolled participants were admitted to an inpatient research unit for an eight-day detoxification and XR-naltrexone induction (Study Day 1). Participants were stabilized on buprenorphine (4 mg bid, Day 2), followed by an opioid washout (Days 3 and 4), and then increasing daily doses of naltrexone (Day 5: 3.125 mg, Day 6: 6.25 mg, Day 7: 25 mg) followed by an injection of XR-naltrexone (Day 8: Vivitrol 380 mg i.m.) and discharge on Day 9. Naltrexone was given once daily at 10 AM. On Day 2, participants began taking study medication (dronabinol or placebo) titrated to 30 mg/day by Day 4. Precipitated withdrawal symptoms were treated with fixed doses (as tolerated) of clonidine (0.8 mg/d), clonazepam (Day 1–6: 3.5 mg/d, Day 7: 2 mg/d, Day 8: 0.5 mg/d), zolpidem 10 mg/d, and other adjuvant medications. Participants were offered zolpidem 5 mg as needed in the first two weeks following discharge from the inpatient unit.
Study medication, dronabinol or placebo, were given under double-blind conditions and dronabinol was administered twice daily at 10 AM and 9 PM with the following schedule of titration: Study Day 2: 10 mg in AM, Study Day 3: 10 mg in AM and PM, Study Day 4 onward: 15 mg in AM and PM. Dose selection was guided by the prior clinical trial’s experience with dronabinol, the need to test the sufficiently high dose of dronabinol to test study’s primary hypothesis, and tolerability that will permit its use on an outpatient basis in individuals who are intolerant to marijuana (Budney et al., 2007; Haney et al., 2008; Levin et al., 2011).
Following discharge, participants received outpatient treatment for 8 weeks, attending the clinic three times per week. Participants continued with dronabinol or placebo for 5 outpatient weeks. Medication and matching placebo tablets were encapsulated with riboflavin 25 mg to assess compliance. The distribution of daily dose was adjusted, with most patients preferring 20 mg or 30 mg to be taken all at night. The medication was continued at the maximum tolerated dose for the first three weeks, with a gradual dose reduction during weeks 4 (20mg/day) and 5 (10mg/day). Participants received an additional injection of XR-naltrexone at week 4. Medication was tapered off after the second naltrexone injection as the most of withdrawal symptoms were expected to be resolved by then and to limit the possibility that participants will become physically dependent on dronabinol.
During each visit, participants gave an observed urine specimen and completed self-report measures of drug use, craving, and mood. All urine specimens were tested on-site for opioids (morphine, oxycodone, methadone, buprenorphine), psychostimulants, and benzodiazepines, and one sample per week was sent to the laboratory for confirmation testing. All urine samples were tested at the laboratory for THC to preserve blinding to study medication. A research nurse obtained vital signs and assessed side-effects. Medication compliance was assessed at each visit using a structured calendar-based interview and confirmed using a visual inspection of the sample under UV light for riboflavin fluorescence. Participants met with a research psychiatrist once per week to monitor treatment progress and review medication tolerability and adherence. Participants were compensated $15 each visit. Those who stopped dronabinol/placebo for at least two weeks, were classified as study drop-outs.
Participants were asked to attend one weekly individual therapy session that included elements of Motivational Interviewing, Relapse Prevention, and Cognitive Behavioral Therapy. Sessions were audio-taped for supervisory and adherence purposes, and we conducted weekly supervision sessions to prevent therapeutic drift. After completion of study procedures participants were referred to continue treatment in community programs either with naltrexone or buprenorphine, where appropriate.
Assessments and Data Analysis
The primary aim was to compare the severity of opioid withdrawal across the two treatment arms during the eight-day inpatient phase, and during the eight-week outpatient phase. We used the Subjective Opiate Withdrawal Scale (SOWS; Handelsman et al., 1987) collected midday daily during the inpatient phase (approximately 3 hours after the morning dose of study medications and naltrexone) and weekly afterwards. The SOWS includes 16 common symptoms of withdrawal rated on a severity scale from 0=not at all to 4=extremely). In addition, we used the Hamilton Rating Scale for Depression (HAM-D 21; Williams, 1988), an observer rating scale that assesses severity of symptoms emerging during protracted withdrawal (depression, anxiety, vegetative symptoms). HAM-D was collected at baseline and weekly during the trial. Retention in treatment (time to drop out) was assessed for the inpatient phase and the 8-week outpatient phase. Secondary outcomes included weekly proportion of participants who used opiates (dichotomous) and weekly proportion of participants who had cravings (defined as any craving score > 0 during the week; dichotomous). As a covariate we included a marijuana-use status during the outpatient phase (regular use at least once every week throughout trial, dichotomous).
The severity of opioid withdrawal during the inpatient phase was analyzed longitudinally using a generalized linear model with AR(1) structure. The two-way interaction between time (i.e., day) and treatment was assessed first and retained in the final model if found significant. If no significant interaction was found, a model with only main effects of time and treatment was fit. An AR(1) covariance structure was used to account for the correlation of the repeated observations within subjects. Retention rates were compared using Kaplan-Meier Curves and log-rank statistics. To examine the covariate (marijuana use during the outpatient phase), a Cox Proportional Hazards Model was used with treatment and regular marijuana use as covariates. If either was found to be significant, hazard ratio estimates and corresponding confidence intervals were obtained. PROC PHREG in SAS was used to conduct the analysis.
Longitudinal secondary outcomes were analyzed during the outpatient phase using longitudinal generalized, mixed effects models with either identity or logit link functions, depending whether the outcome was continuous or dichotomous. Each HAM-D item was modeled as a dichotomous (=0 or not) outcome modeling the probability of the presence of the individual item. Random intercept and/or autoregressive AR(1) covariance structure were used. The two-way interaction between time and treatment was assessed first and retained in the final model if found significant. If no significant interaction between time and treatment was found, a model with only main effects was fit. Analysis of HAM-D was also adjusted by baseline. PROC GLIMMIX in SAS® was used to conduct all secondary analyses. All analyses were intent-to-treat and with two-tailed alpha and significance level of 5%, unless otherwise stated.
RESULTS
Sample Characteristic
Participants were on average 38 years of age (SD 11.3), mostly male (85%), and primarily White (58%) or Hispanic (30%). Participants reported using an average of 10 bags of heroin per day (SD 7.6), 50% were injecting heroin and 16% were using prescription opioids (Table 1). Eleven participants (18%) were regular smokers of marijuana (smoked at least once weekly by self-report confirmed with THC positive urine toxicology) prior to study enrollment. Those who smoked marijuana prior to study enrollment were doing it on average 15 (SD 9.4) out of the previous 30 days with an average of 1.5 (SD 0.56) joints per using day. These participants were significantly younger 29.7 yrs (SD 7.7) as compared to the non-smokers 40.9 (SD 12.7) but there were no other demographic differences between these groups.
| Placebo | Dronabinol | ||
|---|---|---|---|
| Characteristic | (n=20) | (n=40) | p-value |
| mean (SD) or n (%) | |||
| Age (years) | 37.3 (11.1) | 38.5 (11.6) | 0.70 |
| Male | 16 (80.0) | 34 (87.2) | 0.47 |
| Race/Ethnicity | 0.89 | ||
| White | 12 (60.0) | 23 (57.5) | |
| Black | 2 (10.0) | 3 (7.5) | |
| Hispanic | 5 (25.0) | 13 (32.5) | |
| Other | 1 (3.7) | 1 (2.5) | |
| SOWS at baseline | 20.3 (16.5) | 20.9 (17.7) | 0.90 |
| STAI at baseline | 26.3 (14.7) | 24.7 (11.4) | 0.66 |
| HAMD at baseline | 7.5 (4.8) | 7.5 (5.1) | 0.99 |
| Pattern of opioid use at baseline | |||
| Route | 0.96 | ||
| IV (heroin) | 10 (50.0) | 20 (50.0) | |
| IN (heroin) | 7 (35.0) | 13 (32.5) | |
| Average daily use (bags heroin) | 9.5 (5.0) | 10.3 (8.7) | 0.65 |
| PO (prescription opioids) | 3 (15.0) | 7 (17.5) | |
| Average daily use (mg oxycodone) | 110.3 (61.0) | 152.7 (190.7) | 0.72 |
| Duration of opioid use (years) | 10.0 (6.2) | 12.2 (11.4) | 0.45 |
| Baseline drug and alcohol use | |||
| Any marijuana use at baseline | 5 (25.0) | 10 (25.0) | 1.00 |
| Cocaine | |||
| Any use in last 30 days | 2 (10.0) | 14(35) | 0.039 |
| Alcohol | |||
| Any use in last 30 days | 10(50.0) | 18(45.0) | 0.71 |
*Frequencies may not sum to N=60 due to missing values. Three patients did not report their STAI at baseline. One subject was transgender. Percentages may not add up to 100 due to rounding.
During the outpatient phase of the trial, 32% of participants (N=12) (26% in dronabinol and 36% in placebo) smoked marijuana regularly. Marijuana smoking was more frequent among patients who were regular (at least weekly) marijuana users prior to the study, compared to those who were not using before study entry (89% vs.15%). During outpatient treatment phase participants who smoked marijuana were doing so on average 18.1 days/month (SD=10.9, range 1–30). There was no significant increase in the frequency of smoking for participants who continued to smoke after discharge from the inpatient unit.
Opioid withdrawal
Figure 2 shows the observed means of the SOWS scores, reflecting opioid withdrawal severity, at baseline (Day 1), and daily across the inpatient treatment period (days 2 through 8) during which study medication (dronabinol vs placebo) was administered. All patients were receiving a standard rapid detoxification consisting of low dose buprenorphine on Day 2 only and non-opioid ancillary medications thereafter with oral naltrexone titration beginning on Day 5.
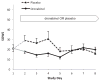
As can be seen in the Figure 2, participants presented for treatment on Day 1 with generally low to moderate levels of baseline withdrawal severity and no significant group difference was observed at Day 1 - placebo: 20.3 (SD 16.5); dronabinol 20.9 (SD 17.7) (SOWS: out of 64 maximum score, see Figure 2). During days 2 trough 4 (before oral naltrexone was introduced), the observed withdrawal scores are higher in the placebo group than the dronabinol group. During days 5 to 8 (after the introduction of naltrexone), the withdrawal scores for the groups appear to converge. The observed data in Figure 2 suggest possibly a meaningful effect of dronabinol on SOWS during days 2 to 4 (before naltrexone was introduced) that washes out during days 5 to 8. The average difference between placebo and dronabinol during days 2–4 was 11.34 and during days 5–8 was 6.69. Even though the difference in SOWS when only modeled for days 2–4 is significant (T28 = 3.26, p = .003) and not significant when only modeled for days 5–8 (T28 = 1.54, p = .13), the overall analyses didn’t find the differences between days 2–4 and 5–8 significant. Notice the wide overlapping confidence intervals for days 2–4 (95% CI: 4.51 – 18.17) and 5–8 (95% CI: −1.81–15.19) suggesting that the observed differences in SOWS severity rating before and after naltrexone introduction could be potentially Type I Error. This is also supported by two additional analyses.
First, when treatment (naltrexone vs. placebo), study day (2–8), and the interaction between treatment and study day were entered into the generalized linear model, the interaction between study day and treatment was not significant (F6,124 = 0.93, p = 0.48) suggesting that there are no significant differences between effect of naltrexone and placebo over time. After the interaction was removed from the model there was a significant main effect of treatment (F1,55 = 8.80, p = 0.005), with an estimated severity in placebo group at 26.5 and in dronabinol group at 16.7 across days 2–8 (average estimated difference was 9.8), with no significant effect of day in a main-effects model.
A second analysis was run to statistically quantify the observed differences between dronabinol and placebo over days 2–4 compared to the effect during days 5–8. First, the study days were categorized as part of 2 time phases (Phase 1 included days 2–4; Phase 2 included Days 5–8). Then a mixed effect longitudinal model was fit with treatment, interval (Phase 1: before naltrexone vs. Phase 2: after naltrexone), and the treatment by phase interaction. The interaction between the treatment and phase, similarly to previous model, did not reach significance (F1,28=1.19, p=.28) suggesting that the differences between phase 1 and phase 2 are not statistically significant even though appearing clinically meaningful. With the interaction removed from the model, the overall (days 2–9) estimated difference between placebo and dronabinol is 9.9 points (T=3.04, p=0.004) that is significant. Thus, although the observed data suggest that the observed and clinically meaningful effect of dronabinol occurs mainly during days 2 to 4 (before naltrexone in introduced) and that the effect of dronabinol washes out during days 5 to 8, the statistical models indicate a statistical inability to detect a significant treatment by time, or treatment by phase interaction. This indicates that the observed data do not provide strong enough evidence to say that the only significant effect of dronabinol can be found only during days 2–4. The 95% confidence intervals suggests that potentially the true difference between placebo and dronabinol can be as small as 4.51 points during days 2–4 and as high as 15.19 points during days 5–8. It is a clear that the clinically meaningful difference between days 2–4 and days 5–8 needs stronger statistical support (larger number of subjects in future studies) to be found significantly different.
We conducted a moderator analysis to assess whether the treatment effect on opioid withdrawal while inpatient was different for participants who smoked marijuana vs. non-smokers (by adding an interaction term between treatment and smoking status into the model). There was no effect of pre-enrollment marijuana smoking on the severity of SOWS-rated opioid withdrawal during inpatient phase (F1,48 < 0.01, p = 0.96).
For the outpatient phase, there was a significant effect of week with withdrawal severity decreasing (F7,163=36.72, p <0.0001) in the main-effects model. Week by treatment interaction was not significant and there were no significant effects of treatment groups or post-detoxification marijuana use.
Retention in treatment
Of the 60 participants who entered the study, 38 (63%) completed the inpatient phase, received the injection of XR-naltrexone and continued in the outpatient phase. There was no significant difference in the rate of successful induction onto XR-naltrexone between dronabinol (66%) and placebo (55%) groups (X2 = 1.46, p = 0.23). Pre-enrollment marijuana use was not a significant predictor of inpatient treatment retention (X2 = 1.45, p = 0.23). Of the 38 participants who entered the outpatient phase, 82% completed at least 4 weeks of treatment, and 55% completed all 8 weeks of the trial. No significant difference was found in retention between the placebo and the dronabinol groups, with 35% retained in both groups.
When post-detoxification marijuana use was entered as a covariate in the model, there was a significant effect of marijuana use on outpatient treatment retention (X2= 4.31, p = 0.038) with marijuana smokers more likely to remain in treatment as compared to non-smokers (Hazard rate = 4.83, 95% CI: 1.09, 21.36 while controlling for treatment arm; Figure 3). All of those who were using marijuana remained in treatment and received the second XR-naltrexone injection as compared to only 46% of participants who were not using marijuana. Because marijuana smokers were significantly younger we entered age into the treatment retention model but the effect of marijuana use on treatment retention remained relatively unchanged suggesting that younger age did not explain the relationship between marijuana use and retention.

Secondary outcomes
For the HAM-D outcome, interaction between treatment and week was not significant and was omitted. The main effect of week was significant, with HAM-D severity decreasing over time (F7,151 = 9.71, p <0.0001). Positive indicator of marijuana use was significantly associated with lower total HAM-D scores while controlling for baseline severity, week, and treatment (F1,151=4.43, p = 0.037). The main effect of marijuana smoking during the study was significant for dichotomized outcomes: insomnia (early, middle, and late) and anxiety (psychological). Marijuana smokers had lower odds of having these symptoms compared to non-smokers.
While outpatient, 63% of participants used opioids at least on one occasion. The interaction between treatment and study week was not significant and was omitted. Only main effect of week (F7,193=7.22, p <0.01) was significant; with 10% of participants using opioids during the last week of trial. There were no significant main effects of treatment or post-detoxification marijuana use. For the craving outcome, there was no significant interaction between treatment and study week, but there was a significant effect of week (F7,167=2.59, p =0.015), with craving decreasing over time. There were no main effects of treatment and post-detoxification marijuana use.
Medication compliance and safety
Medication compliance was quantified as the proportion of days in which 80% or more of capsules were taken. No significant difference in rates of compliance between the placebo and dronabinol groups (80% and 84%) was found. The mean maximum tolerated dose of study medication (of the maximum three dronabinol 10 mg capsules or placebo per day) was 2.9 ± .3 capsules/day in the placebo group, 2.7 ± .6 capsules/day in the dronabinol arm. Most participants took all doses of adjunctive medications during the inpatient and early outpatient phase, and there was no difference between treatment groups in the amount of adjunctive medications used.
We have measured urine concentration of THC during the outpatient phase of the study. There was a significant effect of treatment with participants assigned to dronabinol had over 900% higher levels of THC compared to subjects assigned to placebo, while controlling for marijuana use status and time (F1,99=33.4, p <0.0001). There was also a significant effect of marijuana use on urine THC concentration with participants smoking marijuana having 130% higher THC values compared to non-smokers while controlling for treatment and time (F1,99=5.65, p<0.02). These results suggest that dronabinol had greater impact than marijuana smoking on average urine THC concentration.
Adverse effects (AEs) were reported by 91% of the placebo group and 96% of the dronabinol group (Table 2). The most commonly reported side effects were insomnia (placebo 64%, dronabinol 63%), nausea/vomiting (placebo 18%, dronabinol 18%), diarrhea (placebo 18%, dronabinol 26%) consistent with symptoms of naltrexone-related protracted withdrawal. Other frequent AEs were mood changes (placebo 36%, dronabinol 18%) and fatigue/drowsiness (placebo 9%, dronabinol 26%). There were no significant differences between treatment groups in frequency of AE reporting. Most side effects were consistent with symptoms of naltrexone-related protracted withdrawal and occurred primarily during the first three weeks of the outpatient treatment and none of them were sustained. None of the participants reported discontinuing the study because of AEs. There were no significant differences between treatment groups at the end of treatment in: weight, blood pressure, heart rate, respiration, and oral temperature. We have also assessed the frequency of AEs between marijuana smokers and non-smokers and there were no significant differences between groups.
| AEs % (n) | ||
| Placebo (N=11) | Dronabinol (N=27) | |
| Number of Participants who were removed from trial because of SAEs* | 0 | 4% (1) |
| Number of Participants with at least 1 AE* | 91% (10) | 96% (26) |
| Number of Participants requiring dose reduction | 9% (1) | 22% (6) |
| Number of Participants requiring discontinuation of medication | 0 | 4% (1) |
| Adverse Effects* | ||
| Insomnia | 64% (7) | 63%(17) |
| Mood Changes | 36% (4) | 18% (5) |
| Increase/decreased appetite | 18% (2) | 7% (2) |
| Fatigue/drowsiness | 9% (1) | 26% (7) |
| Nausea/Vomiting | 18% (2) | 18% (5) |
| Diarrhea | 18% (2) | 26% (7) |
| Headache | 18% (2) | 11% (3) |
| Body Aches | 9% (1) | 11% (3) |
| GI Distress | 27% (3) | 15% (4) |
| Sweating/chills | 0 | 15% (4) |
Three serious adverse events occurred in this study. A participant developed symptoms of pruritus and facial swelling 2 days after receiving an injection of naltrexone. A dermatologist determined that the rash was most likely not related to the study medication, but the participant stayed off the naltrexone for the reminder of the trial. One participant in the placebo arm was hospitalized for symptoms of dehydration during the first week of the outpatient phase and another was hospitalized for treatment of a kidney infection and dislocated shoulder, which occurred 3 weeks after discontinuation of study participation.
DISCUSSION
In this double-blind and placebo controlled clinical trial of a cannabinoid agonist for opioid-dependent patients undergoing treatment with XR-naltrexone, dronabinol (30 mg per day) reduced opioid withdrawal symptoms during the acute inpatient phase of withdrawal and naltrexone initiation. This beneficial effect of dronabinol appeared (based on inspection of the observed data, Figure 2) to occur mainly during the first few days of opioid detoxification, before oral naltrexone began to be introduced, although the treatment by time interaction is not significant. It is also possible that the relatively short duration of opioid-withdrawal limited the detectable effect of dronabinol to the first few days of treatment. There were no significant differences between dronabinol and placebo in rates of adherence with the first or second naltrexone injections or the rates of completing 8 weeks of treatment. Approximately one third of participants in the study smoked marijuana regularly during the outpatient treatment, a behavior that was voluntary and independent of treatment assignment. Those participants had less insomnia and anxiety symptoms and were more likely to remain in treatment, as compared with participants who were smoking marijuana rarely or not at all. The frequency of opioid use and the severity of opioid withdrawal and craving decreased over time but no effect of dronabinol or marijuana use was observed.
The finding of diminished opioid withdrawal with dronabinol is consistent with preclinical studies (Frederickson et al., 1976; Lichtman et al., 2001; Vela et al., 1995; Yamaguchi et al., 2001) and smoked marijuana also appeared to diminish opioid withdrawal in patients undergoing stabilization on methadone (Scavone et al., 2013b). In the present trial, participants were also receiving other medications during the detoxification, so it is not clear if dronabinol by itself would be sufficient to produce clinically significant reduction of opioid withdrawal. Opioid withdrawal affects multiple organs and systems and its management may best accomplished using a combination of medications, targeting different components of the syndrome. We hypothesized that reduction of withdrawal severity would result in higher rates of successful initiation of treatment with injectable naltrexone, but this was not observed.
Most participants continued to experience some insomnia, low appetite, and low energy during the first few weeks of outpatient treatment, a set of symptoms consistent with protracted withdrawal or low-grade withdrawal symptoms related to naltrexone. Unlike the positive effects on acute opioid withdrawal, treatment with dronabinol did not affect these symptoms, as measured by the Hamilton Depression Scale. This suggests either that there might be a threshold of symptom severity necessary for dronabinol to be effective, or that dronabinol is effective at relieving mainly acute opioid withdrawal, rather than subacute or protracted withdrawal or withdrawal related to initial naltrexone treatment. Alternatively, participants developed a tolerance to the effects of dronabinol over the five weeks of its administration. Pharmacokinetic factors might also play a role. Dronabinol has a variable metabolism and low bioavailability (Ben Amar, 2006; McGilveray, 2005) and it does not produce dose-dependent effects (Bedi et al., 2013; Haney et al., 1999), suggesting that blood levels of dronabinol might have been low and inconsistent which might have contributed to the reduced clinical effect. In the present trial many participants elected to take medication once a day at night, however with the dronabinol’s elimination half-life of 19–36 hours, once daily dosing might not be sufficient to provide consistent blood level and medication effect throughout the day. On the other hand, decreasing the frequency of daily dosing increases rates of compliance with medication and evening dosing minimizes the risk of sedation which was experienced by some patients further improving medication compliance. It is also possible that after release from the inpatient unit, participants become less compliant with the medication. Thus lowering the dronabinol blood level might explain its efficacy during the inpatient but not the outpatient treatment phase.
One of the interesting study findings was the observed beneficial effect of marijuana smoking on treatment retention. Approximately one third of participants smoked marijuana regularly during the outpatient treatment phase. Participants who smoked marijuana had less difficulty with sleep and anxiety and were more likely to remain in treatment as compared to those who were not using marijuana, regardless of whether they were taking dronabinol or placebo. This finding is consistent with our earlier reports showing the beneficial effects of moderate and intermittent marijuana use on compliance with naltrexone and opioid abstinence (Church et al., 2001; Raby et al., 2009). Although none of these studies used a controlled design to assess the effect of marijuana smoking a priori, the fact that we were able to replicate the finding in three separate studies strengthens its validity. Moreover, this finding is consistent with our initial hypothesis that cannabinoid agonists may alleviate some of the discomfort present during the initiation of naltrexone treatment and allow patients to remain in treatment and abstinent from opioids. In fact, all of the participants who used marijuana in our study received the second injection of XR-naltrexone, compared with average of 70–80% observed in previous trials using XR-naltrexone (Comer et al., 2006; Krupitsky et al., 2011). Although this is an uncontrolled observation based on a small sample of patients, it further supports the hypothesis that cannabinoid agonists might be a useful adjunct to antagonist-based treatment of opioid dependence. It has to be noted however that THC is only one among many biologically active alkaloids in marijuana and other compounds might be more critical to the anti-withdrawal effect of smoked marijuana. Interestingly, in the present trial dronabinol was more likely than marijuana to increase average level of urinary THC, which suggests that perhaps non-THC constituents of marijuana might have helped with treatment retention. On the other hand self-administration of smoked marijuana at specific time points and in response to distress, resulted in pulsatile high doses of THC that helped with emerging symptoms.
As smoked marijuana is not currently approved as a medical treatment, alternative compounds such as pharmaceutical extract from a marijuana plant or an alternative cannabinoid medication with better pharmacokinetics might be better suited to decrease the severity of acute opioid withdrawal and reduce early relapse to opioid use. However, the attempt at maximizing the potency and effectiveness of medication delivery has to be weighed against the adverse effects profile and potential for abuse. In the present trial participants who smoked marijuana included mostly individuals who were smoking prior to study entry, and there is no indication that exposure to dronabinol increased the risk of marijuana smoking initiation. Even though earlier studies did not show a negative impact of marijuana use on treatment retention in agonist-based treatment of opioid dependence (Epstein and Preston, 2003), a large clinical trial of opioid agonist maintenance conducted recently showed a negative effect of marijuana use on treatment outcome (Hser et al., 2013). This apparent differential effect of smoked marijuana on treatment outcome suggested that marijuana use might be more destabilizing for patients in agonist vs. the antagonist-based treatment.
Overall, dronabinol appeared to be well tolerated in this population; frequency of adverse effects and requests to lower medication were comparable between dronabinol and placebo groups. The majority of participants complained of adverse effects during the first 1–3 weeks following discharge from the inpatient unit but it is difficult to distinguish withdrawal symptoms from the side effects of dronabinol (e.g., sedation). Requests for dose reduction of medication occurred with equal frequency in the medication and the placebo groups, suggesting that it was primarily withdrawal that was responsible for these adverse effects. Compliance with study medication appears to be good by self-report confirmed by UV fluorescence and significantly higher average levels of THC in participants assigned to active medication condition.
Study limitations include a relatively small sample size. It seems unlikely that limited power prevented us from detecting an effect of dronabinol on study retention, and power was sufficient to detect the effect of marijuana smoking status. The failure to detect an interaction between treatment and time on withdrawal symptoms during the inpatient phase might reflect limited power. A single dose of dronabinol was a limitation as higher dose might have been effective during the outpatient phase of the trial. Blood rather than urinary THC levels might have been a better measure of medication compliance. Uncontrolled smoking of marijuana by some participants introduced a confounding variable as marijuana contains THC, a compound under study in this trial. Finally, a large number of interested participants were excluded due to medical or psychiatric instability, limiting the generalizability of study findings.
In conclusion, this double-blind controlled trial demonstrated that dronabinol reduced the severity of opiate withdrawal during opioid detoxification and rapid induction onto XR-naltrexone. This effect of dronabinol appeared to occur mainly during the first few days of opioid detoxification, before oral naltrexone was introduced, although the treatment by time interaction is not significant. Dronabinol did not significantly improve either low-grade withdrawal symptoms during subsequent outpatient treatment with naltrexone, nor the proportion of patients inducted onto and maintained on injection naltrexone. Naturalistic marijuana smoking was associated with improved retention on injection naltrexone. Although the finding on naturalistic marijuana smoking suggests cannabinoid agonists may be useful for improving retention on treatment with injection naltrexone, the findings do not support the effectiveness of dronabinol on retention. Further research is warranted on other cannabinoids for facilitating treatment with naltrexone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. 2013;18:872–881. [PMC free article] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Carpenter KM, Mariani JJ, Levin FR, Nunes EV. A placebo-controlled trial of memantine as an adjunct to injectable extended-release naltrexone for opioid dependence. J Subst Abuse Treat epub Jan. 2014;21:2014. [PMC free article] [PubMed] [Google Scholar]
- Brooks AC, Comer SD, Sullivan MA, Bisaga A, Carpenter KM, Raby WM, Yu E, O’Brien CP, Nunes EV. Long-acting injectable versus oral naltrexone maintenance therapy with psychosocial intervention for heroin dependence: a quasi-experiment. J Clin Psychiatry. 2010;71:1371–1378. [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. [PubMed] [Google Scholar]
- CDC. CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- Church SH, Rothenberg JL, Sullivan MA, Bornstein G, Nunes EV. Concurrent substance use and outcome in combined behavioral and naltrexone therapy for opiate dependence. Am J Drug Alcohol Abuse. 2001;27:441–452. [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98:269–279. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders - patient edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Frederickson RC, Hewes CR, Aiken JW. Correlation between the in vivo and an in vitro expression of opiate withdrawal precipitated by naloxone: their antagonism by l-(−)-delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1976;199:375–384. [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197:157–168. [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999;141:385–394. [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2013;109:79–87. [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- Lopez-Moreno JA, Gonzalez-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addict Biol. 2008;13:160–187. [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. [PubMed] [Google Scholar]
- McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl A):15A–22A. [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am J Drug Alcohol Abuse. 2006;32:503–517. [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther. 2013;138:84–102. [PMC free article] [PubMed] [Google Scholar]
- Parolaro D, Rubino T, Vigano D, Massi P, Guidali C, Realini N. Cellular mechanisms underlying the interaction between cannabinoid and opioid system. Curr Drug Targets. 2010;11:393–405. [PubMed] [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. [PMC free article] [PubMed] [Google Scholar]
- Raby WN, Carpenter KM, Rothenberg J, Brooks AC, Jiang H, Sullivan M, Bisaga A, Comer S, Nunes EV. Intermittent marijuana use is associated with improved retention in naltrexone treatment for opiate-dependence. Am J Addict. 2009;18:301–308. [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Berrendero F, Ozaita A, Maldonado R. Advances in the field of cannabinoid--opioid cross-talk. Addict Biol. 2008;13:213–224. [PubMed] [Google Scholar]
- SAMHSA; ISubstance Abuse and Mental Health Services Administration, editor. An Introduction to Extended-Release Injectable Naltrexone for the Treatment of People with Opioid Dependence. SAMHSA Advisory; Rockville, MD: 2012. [Google Scholar]
- SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary Of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013a;248:637–654. [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling RC, Weinstein SP, Van Bockstaele EJ. Impact of cannabis use during stabilization on methadone maintenance treatment. Am J Addict. 2013b;22:344–351. [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132:215–241. [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse. 2012;38:187–199. [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–653. [PubMed] [Google Scholar]
- Vela G, Ruiz-Gayo M, Fuentes JA. Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology. 1995;34:665–668. [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. [PubMed] [Google Scholar]
- Yamaguchi T, Hagiwara Y, Tanaka H, Sugiura T, Waku K, Shoyama Y, Watanabe S, Yamamoto T. Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res. 2001;909:121–126. [PubMed] [Google Scholar]
This article originally appeared at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4536087/