The Influence of Recency of Use on fMRI Response During Spatial Working Memory in Adolescent Marijuana Users
This originally appeared at https://pubmed.ncbi.nlm.nih.gov/21053763/
Abstract-
Some neurocognitive recovery occurs within a month of abstinence from heavy marijuana use, yet functional magnetic resonance imaging (fMRI) has revealed altered activation among recent and abstinent adult users. We compared fMRl response during a spatial working memory (SWM) task between adolescent marijuana users with brief and sustained durations of abstinence. Participants were 13 recent users (two to seven days abstinent), 1 3 abstinent users (27 to 60 days abstinent), and 1 8 nonusing controls, all ages 1 5 to 1 8. Groups were similaron demographics, had no psychiatric or medical disorders, and user groups were similar on substance histories. Teens performed a two-back SWM task during fMRI. Recent users showedgreaterfMRI response in medial and left superior prefrontal cortices, as well as bilateral insula. Abstinent users had increased response in the right precentral gyrus (clusters <::1 328 fll, p < .05). Results suggest that adolescents who recently used marijuana show increased brain activity in regions associated with working memory updating and inhibition. This study preliminarily suggests that ( I ) recent marijuana use may disrupt neural connections associated with SWM and result in compensatory brain response, and (2) sustained abstinence from marijuana may be associated with improvements in SWM response among adolescents
Regular marijuana use is prevalent among teenagers (Johnston et al. 2006). However, it remains unclear whether potential neural disruption associated with adolescent marijuana use is permanent, or whether alterations improve with abstinence, raising the possibility of neurocognitive recovery. Functional neuroimaging may help differentiate the neurocognitive effects ofrecent use from the potentially persisting impact of heavy marijuana use in adolescence.The adult literature indicates impairments in learning and memory, attention, visuospatial skills, processing speed, and executive functioning among heavy marijuana users within afew days of use (Lyons et al. 2004; Bolla et a!. 2002;
Solowij et a!. 2002; Croft et a!. 200 1 ; Pope et a!. 1 997; Pope & Yurgelun-Todd 1 996; Varma et a!. 1 988). Longitudinal evidence suggests that marijuana-related verbal learning deficits, observed following recent use, disappear after 28 days of abstinence in a sample of adults (Pope et a!. 200 1 ). In contrast, compared to published test norms, 28-day abstinent adults demonstrated memory, executive functioning, and motor impairments that correlated with lifetime use (Bolla et a!. 2002). A functional magnetic resonance imaging (fMRI) study examined visuospatial attention among 1 2 recent marijuana users who had used two to 24 hours before scanning, 1 2 abstinent users who had been abstinent an average of 38 months, and 19 nonusing controls (Chang et a!. 2006). Active users demonstrated greater levels of functional abnormalities than abstinent users in prefrontal and cerebellar regions, and a longer duration of abstinence was associated with normalization of function.The question of whether cognitive abilities change with abstinence in adolescents has begun to be explored. Marijuana-dependent youths showed poorer memory performance relative to control youths at treatment intake, and failed to show improvements in short term memory following six weeks of abstinence (Schwartz et a!. 1 989). In a cross-sectional study of youths, current heavy users showed deficits in immediate and delayed memory,
processing speed, and overall IQ, yet three-month abstinent users performed similarly as controls (Fried, Watkinson & Gray 2005). Others have found fMRI evidence of altered working memory activation among abstinent adolescent marijuana users (Schweinsburg et a!. 2008; Jacobsen et a!. 2007, 2004). However, as participants were not assessed soon after marijuana discontinuation, it is unclear whether observed abnormalities represented changes in functioning during early abstinence. We previously identified altered fMRI response to a spatial working memory (SWM) task among adolescents with comorbid marijuana and alcohol use disorders after an average of eight days of marijuana abstinence (Schweinsburg et a!. 2005). Thus, altered functioning has been described in both recent and abstinent adolescent marijuana users, particularly on working memory tasks. However, it remains unclear whether neural activation patterns might change during the first month of abstinence, as recent and abstinent users have not yet been compared with fMRI.This study was designed to investigate the differences in neural activation between recent and abstinent adolescent marijuana users. Evidence in adults suggests that substantial neuropsychological recovery appears to occur after the first week of abstinence, and full normalization is observed following one month of abstinence (Pope et a!. 200 1 ).
Therefore, in order to best characterize the persisting vs. residual effects of marijuana, we examined fMRI response during a SWM task among: ( I ) recent marijuana using teens who had used within one week of testing, (2) abstinent marijuana using teens who had not used for 27 days, and (3) demographically similar nonusing controls. Blood oxygen level-dependent (BOLD) fMRI was collected during a SWM task that activates bilateral dorsolateral prefrontal and posterior parietal networks in adolescents (Schweinsburg, Nagel & Tapert 2005), and has been associated with neural dysfunction among youths with alcohol use disorders (Tapert et a!. 2004) as well as marijuana abuse (Schweinsburg et a!. 2008, 2005). Based on our previous findings, we predicted that recent users would show increased levels of frontal lobe fMRI activation compared to abstinent users, indicating compensatory neural recruitment.
METHOD
Participants
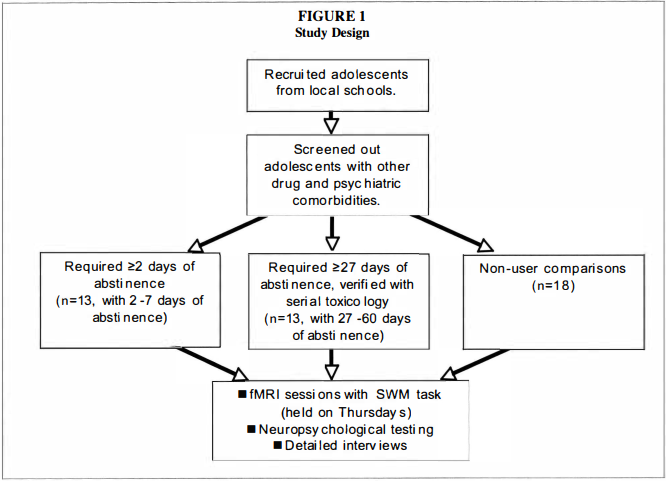
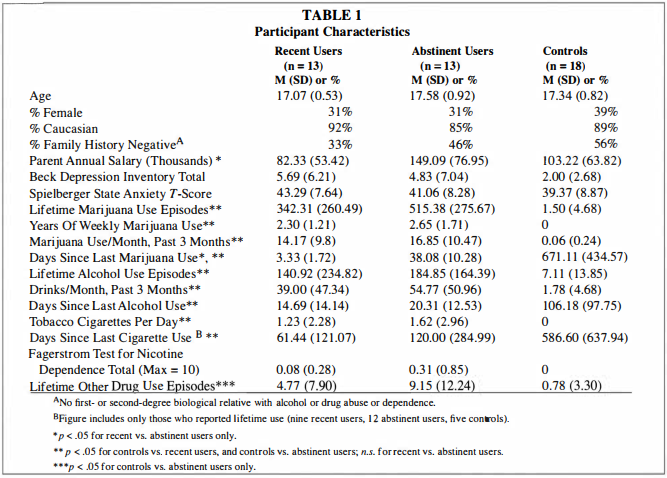
Participants were selected from adolescent brain imaging studies of 15- to 1 8-year-olds recruited from local high schools (Tapert et a!. 2007, 2004, 2003). Teens and their parents were individually screened with telephone interviews; informed consent and assent (for minors), approved by the University of California Human Research Protections Program, were obtained from all participants and their parents. Exclusion criteria included left-handedness; history of neurological disorder or head injury with loss of consciousness for more than two minutes; history of an Axis I psychiatric disorder (including attention-deficit hyperactivity disorder) other than alcohol or marijuana use disorders or conduct disorder; current psychotropic medication use; family history of bipolar I or psychotic disorders; prenatal alcohol or other drug exposure; learning disabilities; and MRI contraindications. Teens with alcohol use disorders or conduct disorder (one control, three recent users, and two abstinent users) were not excluded due to high comorbidity with heavy marijuana use (Agosti, Nunes & Levin 2002; Brown et al. 1 996).Previous studies in adults have suggested considerable neurocognitive recovery following a week of abstinence from marijuana use, and complete normalization after 28 days (Pope et a!. 200 I ). Therefore, the current study examined marijuana users in two groups: 13 recent users who had used within one week of scanning (abstinent two to seven days), and 1 3 abstinent users who had been abstinent between 27 and 60 days. Nonusing control adolescents (n = 1 8) were included to aid interpretation of fMRI differences between the marijuana using groups by identifying brain regions typically activated by this SWM task among demographically similar healthy youths (see Figure 1 for a graphic of the study design). We previously characterized fMRI response to SWM among nonusing controls and abstinent marijuana users (Schweinsburg et a!. 2008). Some of those participants were also included in the current study, but the recent and abstinent marijuana users here have not yet been directly compared. All three groups herein were similar in gender and ethnic composition, estimated premorbid IQ, socioeconomic status, mood, and behavioral indices (see Table 1 ). Importantly, recent and abstinent marijuana users were comparable on all substance use characteristics (including alcohol use) other than recency of marijuana use. As expected, marijuana groups demonstrated greater involvement with alcohol and marijuana than controls. Abstinent marijuana users reported more lifetime uses ofother drugs than controls, but average lifetime use was relatively limited in all groups.
Measures
Behavioral characteristics. Lifetime and recent substance use were characterized with the Customary Drinking and Drug Use Record (CDDR; Brown et a!. 1 998). The Beck Depression Inventory (Beck 1 978) and state scale of the Spielberger State Trait Anxiety Inventory (Spielberger, Gorsuch & Lushene 1 970) assessed mood at the time of imaging (see Table 1).Neuropsychological functioning. Visuospatial skills (Block Design), working memory (Digit Span), and estimated premorbid IQ (Vocabulary) were ascertained with age-appropriate subtests from the Wechsler Intelligence Scale for Children-Third Edition (WISC-III; Wechsler 1 993), Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler 1997), or Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999).Spatial working memory task. The SWM task (Kinderrnann et a!. 2004; Tapert et a!. 200 1 ) was administered in a blocked design, with 18 2 1 -second blocks that alternated between working memory, baseline vigilance, and fixation. In the SWM condition, participants viewed abstract line drawings that were sequentially presented in one of eight locations on a screen, and were instructed to press a button every time a figure appeared in the same location as a previous design within that block. Repeat location stimuli were two-back, and three of ten trials in each block were target items. The baseline vigilance condition presented the same abstract stimuli shown in the same possible spatial locations as in the SWM condition, and subjects were to press a button every time a figure appeared with a dot above it (30% of trials). Resting blocks displayed a fixation cross in the center ofthe screen. For working memory and vigilance conditions, stimuli were presented for 1 000 ms with an interstimulus interval of 1 000 ms (2 1 seconds/block; total task time = 7 minutes, 48 seconds).
Procedures
Toxicology screening. Most teens in the abstinent group were selected from an ongoing study involving a biweekly toxicology screening procedure to ensure abstinence from substances for at least 28 days prior to scanning (Tapert et al. 2007). Three teens who did not undergo the 28-day toxicology screening procedure reported that last use was 27 days or more prior to scanning and provided urine screens negative for cannabinoids and other drugs at the scan session. Since these teens were not required to be abstinent and had not received monetary incentive to maintain abstinence for a month before the scan, it is unlikely that information regarding their date of last use would be falsified (Martin et al. 1 996); these participants were thus included in the abstinent group. Based on available toxicology data at the time of scanning, all teens in the abstinent group produced toxicology screens negative for cannabinoids. Teens in the recent use group were asked to be abstinent for at least 48 hours before scanning. Urine toxicology screens were obtained at the time of scanning for eight participants in the recent use group. Five recent users had positive screens, and three recent users produced negative screens despite self-reported use during the week of the scan. Negative screens can be observed as soon as four days following use (Huestis & Cone 1 998); therefore, self-reported use is not inconsistent with urine toxicology results among the three recent users with negative screens.
Imaging protocol.
Anatomical and functional imaging data were acquired using a 1 .5 Tesla General Electric Signa LX scanner. Ahigh-resolution, sagittally acquired structural scan was collected with an inversion recovery prepared T l -weighted 3D spiral fast spin echo sequence (Wong et al. 2000) (TR = 2000 ms, TE = 1 6 ms, FOV = 240 mm, resolution = 0.9375 x 0.9375 x 1 .328 mm, 1 28 continuous slices, acquisition time = 8:36). The functional scan was acquired axially with T2*-weighted spiral gradient recall echo imaging (TR = 3000 ms, TE = 40 ms, flip angle = 90°, FOV = 240 mm, 1 9-21 slices covering the whole brain, slice thickness = 7 mm, reconstructed in-plane resolution = 1 .875 x 1 .875 mm, 1 56 repetitions).
Data Analyses
Demographic, mood, behavioral, and drug use characteristics were compared between groups with ANOVA or chi-square tests as appropriate, and significant differences were followed-up with Tukey all pairwise comparisons. Standardized scores for neuropsychological tests were obtained from published norms, and compared between groups with ANOVA Significant differences were followed-up with Tukey all pairwise comparisons. SWM task performance was examined with repeated measures ANOVAto characterize the main effects of task condition (vigilance vs. SWM), group, and their interaction, both for accuracy and reaction time. Significant interactions were followed up with simple effects t-tests.
Imaging data were processed and analyzed using Analysis of Functional Neurolmages (AFNI; Cox 1 996). Processing began with motion correction of the time series data (Cox & Jesmanowicz 1 999). This created an output file for each participant containing the adjustments made for three rotational and three displacement parameters. Two independent raters inspected the time series data to remove any repetitions on which the algorithm did not adequately adjust for motion or other artifacts. Valid datasets were required to have at least 85% ofrepetitions retained (on average 97% of repetitions were retained for the 44 participants described here). Using a deconvolution procedure (Ward 2002), the time series data were then correlated with a reference function that represented the hypothesized BOLD signal across the time course of the task and modeled expected delays in hemodynamic response (Cohen 1 997). This multiple linear regression approach yielded a fit coefficient that represented the fit between the observed and hypothesized signal for SWM relative to vigilance, while controlling for linear trends and degree of motion correction. Anatomical and functional datasets were warped into standard space (Talairach & Tournoux 1 988), and functional data were resampled into 3.0 mm3 voxels and smoothed with a 5.0 mm full-width half-maximum Gaussian filter.We performed single-sample t-tests for recent users, abstinent users, and controls to determine regions that showed significantly increased or decreased response to SWM relative to vigilance within each group. To control for multiple comparisons in whole-brain data, we used a combination of cluster volume and voxel thresholding (Forman et al. 1 995). Clusters from each single-sample t-test were considered significant if they consisted of 49 contiguous voxels (p < .05) that exceeded 1 328 f.i.l in volume, yielding an overall cluster-wise a = .05 for the whole brain within each group. BOLD response to SWM relative to vigilance was evaluated between abstinent and recent marijuana use groups in a whole-brain independent samples t-test. Again, we controlled for Type I error using the same thresholding technique as described for the single sample t-tests. Clusters were considered significant if they encompassed at least 49 contiguous voxels (t(24) > 2.06,p < .05), yielding significant clusters � 1 328 f.ll at an overall cluster-wise a = .05. In each region demonstrating a group difference, we examined the relationship between BOLD response and marijuana use characteristics as well as potentially confounding factors (e.g., other substance use and conduct disorder) with exploratory regression analyses among the 26 users.
RESULTS
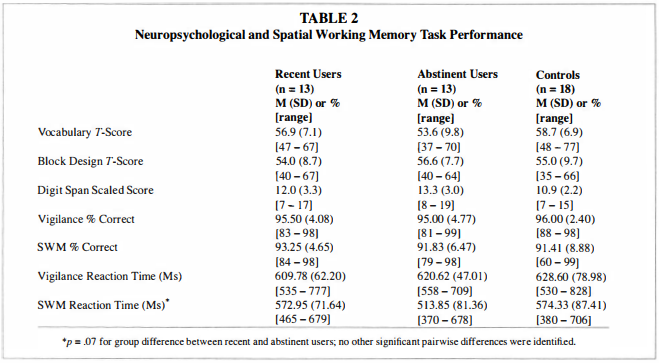
Neuropsychological FunctioningAs shown in Table 2, abstinent and recent users did not significantly differ in Vocabulary [F(2,40) = 1 .5 1 , p < .24], Block Design [F(2,39) = 0.25, p < .78], or Digit Span [F(2,4 1 ) = 2.50, p < . 10] scores. Both marijuana use groups performed similarly to controls on these tests.
SWM Task Performance
Button box logging failed for one recent user, one abstinent user, and one control. Available data revealed a main effect for greater accuracy on vigilance than SWM trials [F( 1 ,38) = 7.33, p = .0 1 ; see Table 2] and no main effect for group or a group x condition interaction for accuracy.
For reaction time, a main effect showed teens were faster on SWM than the vigilance condition [F( l ,38) = 30.76, p < .00 I ] . A trend for a group x condition interaction for reaction time [F(2,38) = 2.87, p = .07] indicated that recent users had slightly faster reaction times for vigilance than abstinent users and controls, and abstinent users showed slightly faster responding than recent users and controls on SWM trials.
Data Quality
Distributions of fMRI response were examined for outliers (Tabachnick & Fidell 2007) in each significant cluster (see Table 3), and none were found. Overall motion was quite minimal, ranging from 0.03 to 0. 1 0 mm for superior-inferior displacement, 0.0 1 to 0. 1 3 mm for left-right displacement, 0.01 to 0.25 mm for posterior-anterior displacement, 0.01 to 0.23· for roll rotation, 0.02 to 0.57" for pitch rotation, and 0.02 to 0. 1 T for yaw rotation. Groups did not differ with regard to discarded repetitions or motion correction applied in any ofthe six motion parameters, except that abstinent users had more head motion in the left-right direction than recent users [t(24) = 2.49; p < .025]. However, such movement did not account for group differences in BOLD response in any region reported below.
fMRI Response
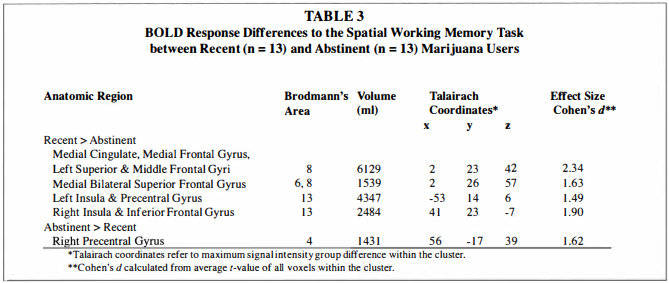
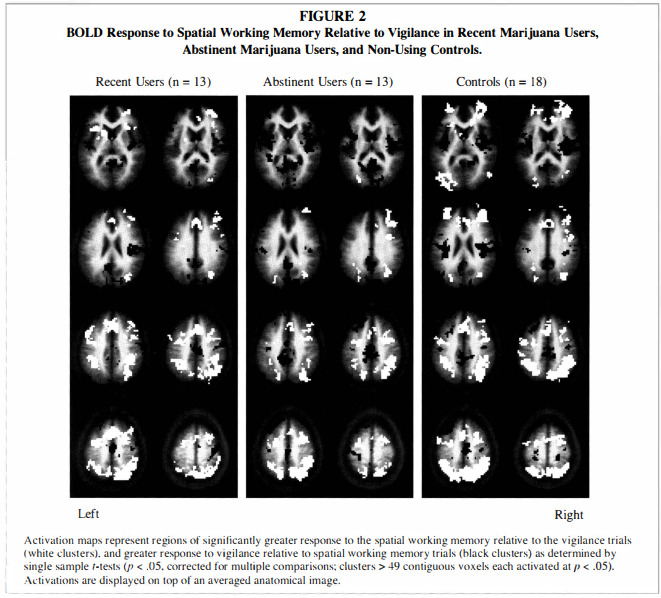
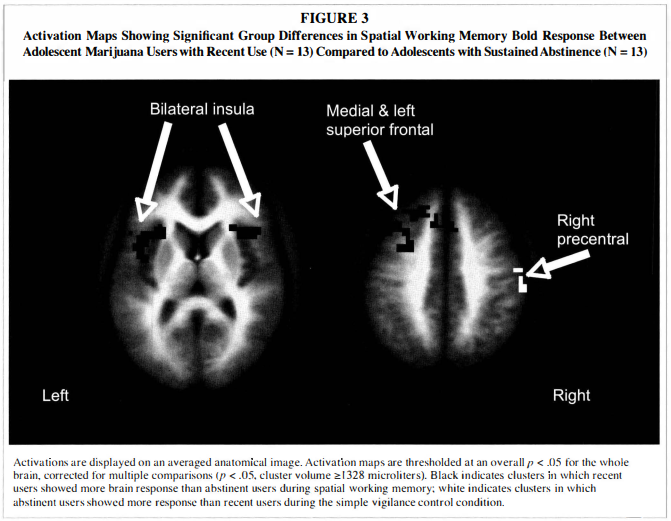
Single sample t-tests showed that the overall pattern of BOLD response was similar in all three groups, with greater SWM activation relative to vigilance in bilateral prefrontal, premotor, cingulate, and posterior parietal areas (p < .05), and reduced SWM response relative to vigilance in medial prefrontal cortex, medial posterior cingulate and cuneus, and several temporal regions (p < .05; see Figure 2).The independent samples t-test revealed that recent users had greater SWM response than abstinent users in the following regions: a large cluster spanning medial bilateral cingulate, medial frontal gyrus, and left superior and middle frontal gyri (Brodmann's area [BA] 8); medial bilateral
superior frontal gyrus (BA 6/8); left insula and precentral gyrus (BA 1 3) ; and right insula and inferior frontal gyrus (BA 1 3 ; see Table 3 and Figure 3). As revealed by single sample t-tests, in frontal and insular regions, recent users demonstrated greater response to SWM than to vigilance, while abstinent users showed reduced response to SWM relative to vigilance, and controls did not show significantly increased or decreased response to SWM relative to vigilance. Abstinent users showed greater BOLD response than recent users only in the right precentral gyrus (BA 4). In this region, recent users demonstrated reduced SWM response relative to vigilance, but controls and abstinent users showed no significant response.To examine the extent to which marijuana use characteristics and potential confounds (e.g., other substance use) might influence observed group differences, we conducted exploratory regression analyses. Of the substance use characteristics listed in Table 1 , no index of marijuana, alcohol, cigarette, or other drug use, except for recency of marijuana use, was related to BOLD response in any region demonstrating a difference between recent and abstinent user groups, and group differences remained significant after controlling for alcohol, nicotine, or other drug use. State anxiety score was positively associated with BOLD response in the left insula/precentral gyrus [F( l ,25) = 4.55, p < .05, R2 = 0. 1 6, b = 0.40]. When anxiety and group (recent vs. abstinent users) were simultaneously entered, group remained a significant predictor of left insula response (p < .005), while anxiety became a predictor at the trend level (p = .05 1 ) . Group differences in BOLD response between recent and abstinent users remained significant after excluding participants with conduct disorder.
DISCUSSION
This study provides a cross-sectional indication of changes in neural response patterns during early abstinence
from marijuana among adolescents. Heavy marijuana using teenagers with two to seven days of abstinence demonstrated greater brain response during spatial working memory than teens who had been abstinent for at least 27 days in medial and left superior prefrontal cortex, and bilateral anterior insula. However, controls did not show significant BOLD response in these frontal and insula regions, suggesting the areas are not normally employed during this SWM task, and may be ancillary regions recruited by recent users to maintain performance levels.Recent users demonstrated enhanced SWM response in medial and left superior prefrontal cortex compared to abstinent users. This region shows strong functional links to posterior parietal cortices (for review, see Wise et al. 1 997), and activation here has been associated with continuous updating and sequencing during spatial working memory (Leung et al. 2007 ; Owen et al. 2005 ; Wager & Smith 2003; Zurowski et al. 2002). In support of this perspective, lesions of the left superior frontal cortex lead to deficits in performance of n-back working memory tasks as executive demands increase, particularly in the spatial domain (du Boisgueheneuc et al. 2006). This region is also activated during uncertainty in decision making (Volz, Schubotz & von Cramon 2005) and appears to have a general role in cognitive control across a variety of tasks (Derrfuss, Brass & von Cramon 2004). Similar to the current study, Chang and colleagues (2006) observed increased visual attention activation in medial frontal regions among adult recent marijuana users relative to users abstinent at least two weeks. Given these prior studies, more effortful cognitive control and working memory updating may correspond with greater superior frontal activation among recent users, particularly if recent users are more uncertain in their responding or if executive demands are high. In contrast, abstinent users may display strategy shifts, requiring less demand on sequencing abilities and general executive control, and task processing may become more efficient with greater lengths of abstinence, eliciting Jess uncertainty in responding.
Bilateral anterior insula/inferior frontal activation was greater in recent users compared to abstinent users. The insula maintains interconnections with both prefrontal and posterior parietal cortices (Selemon & Goldman-Rakic I 988), and is involved in cognitive control and inhibition (Derrfuss, Brass & von Cramon 2004; Wager et al. 2005). Bilateral insula response has been observed across different inhibitory tasks, particularly in relation to task difficulty (Wager et al. 2005). For instance, when performing a response inhibition task, insula activation increases during sleep deprivation, but not after normal sleep, indicating a compensatory role when more effort is required (Chuah et al. 2006). In the context of spatial working memory, the anterior insula has been suggested to assist in selecting appropriate responses and inhibiting irrelevant information (Lepsien et al. 2005). Adult alcoholics perfom1ing a SWM task activated a right inferior frontal region that was more ventral and posterior than activation among controls (Pfefferbaum et a!. 200 I ), and overlapping with the anterior insula region activated by recent users in the current study. The authors suggested that this shift in activation may indicate reorganization of cognitive networks associated with alcoholism. particularly in relation to inhibitory control during SWM. Thus, increased insula response among recent marijuana users may reflect greater inhibitory effort during periods of active marijuana use, whereas abstinent users may no longer require the same degree of inhibitory control. Importantly, the insula may be involved in maintaining protracted substance use patterns. Increased insula activation predicts relapse to substance use among individuals with stimulant dependence (Paulus, Tapert & Schuckit 2005), and cigarette smokers with insular lesions were more likely to maintain abstinence compared to smokers without brain damage (Naqvi et al. 2007). Thus, it could be that the abstinent users in the current study demonstrated reduced insula response because they were able to maintain abstinence forfour weeks prior to scanning; it is unclear whether the recent marijuana users, with their heightened insula activation, would have also been able to remain abstinent for the study. Longitudinal investigations of marijuana users who do and do not relapse within the first month will help clarify this issue.Several possiblemechanisms may subserve the observed results. First, fronto-parietal networks continue structural and functional development throughout adolescence, supporting maturation of working memory abilities (Klingberg 2006; Schweinsburg, Nagel & Tapert 2005; Klingberg, Forssberg & Westerberg 2002; Thomas et a!. 1 999), and may be particularly vulnerable to insult during youth. Cannabinoid receptors are densely located in the frontal cortex (Eggan & Lewis 2007; Glass, Dragunow & Faull 1 997; Herkenham et a!. 1 990). Further, the cannabinoid receptor system develops relatively late (Biegon & Kerman 200 1 ), and receptor densities peak during adolescence among rats (Rodriguez de Fonseca et a!. 1 993), potentially increasing sensitivity to cannabinoid effects in adolescents. Although the neurobiological consequences of chronic cannabinoid exposure are still unclear, alterations of these developing receptor systems may underlie spatial working memory changes among adolescent marijuana users. For instance, chronic marijuana use leads to cannabinoid receptor downregulation in human basal ganglia structures (Villares 2007), which form a frontostriatal network critical to working memory processing (e.g., Chudasama & Robbins 2006). In addition, substantial increases or decreases in dopamine activity lead to impaired working memory performance (for a review, see Robbins 2000). Chronic cannabinoid administration is associated with reduced dopamine functioning in rat prefrontal cortex that lasts at least two weeks following exposure, but begins to normalize with abstinence (Jentsch et al. 1 998; Verrico, Jentsch & Roth 2003). Thus, neurochemical changes that subserve working memory could normalize with sustained abstinence, contributing to the different fMRI activation patterns between recent and abstinent marijuana users.As BOLD response is influenced by cerebral blood flow, it is possible that perfusion data would mediate results reported here. Altered resting frontal and cerebellar blood flow have been observed among recently-using adult chronic marijuana users (Sneider et a!. 2006; Lundqvist, Jonsson & Warkentin 2001 ; Block et al. 2000; Loeber & Yurgelun-Todd 1 999). After a month of abstinence, blood flow velocity and cerebrovascular resistance remained elevated among adult marijuana users, yet those using marijuana at levels consistent with our sample (i.e., 1 5.9 days per month) demonstrated some improvement in vascular resistance (Heming et a!. 2005). The BOLD signal arises from dynamic shifts in cerebral blood flow during activation paradigms (Uludag et a!. 2004; Cohen, Ugurbil & Kim 2002; Hoge et a!. 1 999). Thus, altered BOLD response in adolescent marijuana users could reflect compromised cerebrovasculature. Partial recovery of cerebrovascular perfusion after a month ofabstinence (Heming et a!. 2005) may contribute to fMRI activation differences between abstinent and recent marijuana users in the current study.Abstinent users only demonstrated greater response than recent users in the right precentral gyrus, yet the mechanism behind this group difference is unclear. All participants were right handed and responded with their right index finger during the SWM task, so greater right precentral activation among abstinent users cannot be explained by manual response differences. This increased right precentral response among abstinent users may relate to motor circuitry underlying their faster reaction times, or could be indicative of greater inter-hemispheric motor overflow.
Indices of marijuana use, alcohol use, and other drug use characteristics (including quantity, duration, and frequency of use) were not related to brain response in any region demonstrating a group difference. This indicates that, of the variables examined, only recency of use was related to neural response patterns. This supports the suggestion that recent use may influence brain functioning more than cumulative lifetime exposure (Pope et a!. 200 1 ), and points to the possibility that neural functioning may normalize with longer durations of abstinence.
Although cross-sectional in nature, this study provides preliminary evidence of changes in neural functioning during the first month of abstinence from marijuana. Longitudinal studies, including assessments after differing lengths of abstinence, are needed to clarify the relationship between abstinence duration and neural response. These studies could examine marijuana users who have been abstinent longer than a month to determine whether these brain response differences continue to normalize with extended abstinence. However, group differences in activation may be mediated by characteristics of those users who are able to sustain abstinence for at least 28 days, and may not reflect changes related to recovery per se. Only prospective assessments across an extended abstinence period can determine whether and when shifts in processing occur. Most of the marijuana users in this study were moderate to heavy drinkers. Our previous research characterized neural response differences between adolescent alcohol and marijuana users compared to users of alcohol alone, suggesting that marijuana use may influence neural functioning above and beyond the effects of alcohol, particularly in frontal regions (Schweinsburg et a!. 2005). Indices of alcohol use did not appear to account for group differences in BOLD response found here, yet it is difficult to disentangle the potential neural influence of each substance. Future studies including teens who use alcohol or marijuana alone will help clarify the contributions of each substance and their interaction on brain functioning. Anxiety levels were correlated with brain response in the current study, and although groups did not differ on anxiety, this may be an important factor to consider in later research. Future studies will benefit from larger sample sizes with power to detect neuropsychological differences between groups. Finally, task accuracy was high in both groups; parametric manipulations of working memory load may further differentiate neural response patterns, and may elicit performance decrements in user groups.In summary, heavy marijuana using teenagers who had used within a week of scanning demonstrated greater frontal and insular brain response during SWM than marijuana users who had been abstinent for at least 27 days. Interestingly, such frontal and insular activation was not observed in nonusing control youths, suggesting that recent users recruit additional brain regions not normally utilized forMarijuana in Adolescencethis SWM task. These fMRI differences suggest increased working memory updating and inhibitory control among recent users despite similar task performance. Although cross-sectional, these results could indicate a change in neural strategy during the first month of abstinence, with less reliance on compensatory recruitment of frontal and insular regions. Finally, increased cognitive control and spatial working memory effort following recent use may have important implications for teens attempting abstinence, but longitudinal investigations are needed to verify these initial impressions.
REFERENCES
Agosti, Y.; Nunes, E. & Levin, F. 2002. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. American Journal ofDrug and Alcohol Abuse 28 (4): 643-52.Beck, A.T. 1978. Beck Depression Inventory (BDI). San Antonio, TX:Psychological Corp.Biegon, A. & Kerman, I.A. 200 1 . Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage 14 (6): 1463-68.Block, R.I.; O'Leary, D.S.; Hichwa, R.D.; Augustinack, J.C.; Ponto, L.L.; Ghoneim, M.M.; Arndt, S.; Ehrhardt, J.C.; Hurtig, R.R.; Watkins, G.L.; Hall, J.A.; Nathan, P.E. & Andreasen, N.C. 2000. Cerebellar hypoactivity in frequent marijuana users. Neuroreport I I (4): 749- 53.Bolla, K.l.; Brown, K.; Eldreth, D.; Tate, K. & Cadet, J.L. 2002. Doserelated neurocognitive effects of marijuana use. Neurology 59 (9): 1 337-43.Brown, S.A.; Myers, M.G.; Lippke, L.; Tapert, S.F.; Stewart, D.G. & Vik, P.W. 1998. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal ofStudies on Alcohol 59: 427-38.Brown, S.A.; Gleghorn, A.A.; Schuckit, M.A.; Myers, M.G. & Mott, M.A. 1 996. Conduct disorder among adolescent alcohol and drug abusers. Journal ofStudies on Alcohol 57: 3 1 4-24.Chang, L.; Yakupov, R.; Cloak, C. & Ernst, T. 2006. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 1 29 (Pt 5): 1096- 1 12.Chuah, Y.M.; Venkatraman, Y. ; Dinges, D.F. & Chee, M.W. 2006. The neural basis ofinterindividual variability in inhibitory efficiency after sleep deprivation. Journal ofNeuroscience 26 (27): 7 156-62.Chudasama, Y. & Robbins,T.W. 2006. Functions offrontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological Psychiatry 73 ( I ): 19-38.Cohen, E.R.; Ugurbil, K. & Kim, S.G. 2002. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation leveldependent fMRI response. Journal of Cerebral Blood Flow and Metabolism 22 (9): 1042-53.Cohen, M.S. 1997. Parametric analysis of fMRI data using linear systems methods. Neuroimage 6 (2): 93-103.Cox, R.W. 1996. AFNI: Software foranalysis and visualization offunctional magnetic resonance neuroimages. Computers and Biomedical Research 29: 162-73.Cox, R.W. & Jesmanowicz, A. 1999. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine 42 (6): 1 0 14- 18.Croft, R.J.; Mackay, A.J.; Mills, A.T. & Gruzelier, J.G. 200 1 . The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology 153 (3): 373-79.Derrfuss, 1.; Brass, M. & von Cramon, D.Y. 2004. Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage 23 (2): 604- 1 2.
du Boisgueheneuc, F.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S. & Dubois, B. 2006. Functions ofthe leftsuperior frontal gyrus in humans: A lesion study. Brain 1 29 (Pt 1 2): 3315-28.Eggan, S.M. & Lewis, D.A. 2007. Immunocytochemical distribution of the cannabinoid CB I receptor in the primate neocortex: A regional and laminar analysis. Cerebral Cortex 1 7: 1 75-9 1.Forman, S.D.; Cohen, J.D.; Fitzgerald, M.; Eddy, W.F.; Mintun, M.A. & Noll, D.C. 1 995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use ofa cluster-size threshold. Magnetic Resonance in Medicine 33 (5): 636-47.Fried, P.A.; Watkinson, B. & Gray, R. 2005. Neurocognitive consequences of marihuana - a comparison with pre-drug performance. Neurotoxicology and Teratology 27 (2): 23 1-39.Glass, M.; Dragunow, M. & Faull, R.L. 1 997. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77 (2): 299-3 18.Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R. & Rice, K.C. 1990. Cannabinoid receptor localization in brain. Proceedings ofthe NationalAcademy ofSciences ofthe United States ofAmerica 87 (5): 1 932-36.Heming, R.I.; Better, W.E.; Tate, K. & Cadet, J.L. 2005. Cerebrovascular perfusion in marijuana users during a month ofmonitored abstinence. Neurology 64 (3): 488-93.Hoge, R.D.; Atkinson, J.; Gill, B.; Crelier, G.R.; Marrett, S. & Pike, G.B. 1 999. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magnetic Resonance in Medicine 42 (5): 849-63.Huestis, M.A. & Cone, E. 1998. Urinary excretion half-life of 1 1 -Nor-9- carboxy-09- tetrahydrocannabinol in humans. Therapeutic Drug Monitoring 20: 570-76.Jacobsen, L.K.; Pugh, K.R.; Constable, R.T.; Westerveld, M. & Mencl, W.E. 2007. Functionalcorrelates ofverbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry 61 ( I ): 3 1-40.Jacobsen, L.K.; Mencl, W.E.; Westerveld, M. & Pugh, K.R. 2004. Impact of cannabis use on brain function in adolescents. Annals ofthe New York Academy ofSciences 1 02 1 : 384-90.Jentsch, J.D.; Verrico, C.D.; Le, D. & Roth, R.H. 1 998. Repeated exposure to delta 9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neuroscience Letters 246 (3): 1 69-72.Johnston, L.D.; O'Malley, P.M.; Bachman, J.G. & Schulenberg, J.E. 2006.The Monitoring the Future National Survey Results on AdolescentDrug Use: Overview ofKey Findings, 2005. Bethesda, MD: National Institute on Drug Abuse.Kindermann, S.S.; Brown, G.G.; Zorrilla, L.E.; Olsen, R.K. & Jeste, D.V. 2004. Spatial working memory among middle-aged and older patients with schizophrenia andvolunteers using fMRI. Schizophrenia Research 68 (2-3): 203-16.
Klingberg, T. 2006. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia 44 ( I I ): 2 1 7 1 -7.Klingberg, T.; Forssberg, H. & Westerberg, H. 2002. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience 14 ( I ): 1-10.Lepsien, J.; Griffin, I.C.; Devlin, J.T. & Nobre, A.C. 2005. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. Neuroimage 26 (3): 733-43.Leung, H.C.; Oh, H.; Ferri, J. & Yi, Y. 2007. Load response functions in the human spatial working memory circuit during location memory updating. Neuroimage 35 ( 1): 368-77.Loeber, R.T. & Yurgelun-Todd, D.A. 1 999. Human neuroimaging of acute and chronic marijuana use: Implications for frontocerebeller dysfunction. Human Psychopharmacology Clinical andExperimental 14 (5): 291 -304.Lundqvist, T.; Jonsson, S. & Warkentin, S. 2001 . Frontal lobe dysfunction in long-term cannabis users. Neurotoxicology and Teratology 23 (5): 437-43.Lyons, M.J.; Bar, J.L.; Panizzon, M.S.; Toomey, R.; Eisen, S.; Xian, H. & Tsuang, M.T. 2004. Neuropsychological consequences of regular marijuana use: A twin study. Psychological Medicine 34: 1 239-50.Martin, C.S.; Kaczynski, N.A.; Maisto, S.A. & Tarter, R.E. 1996. Polydrug use in adolescent drinkers with and without DSM-IV alcohol abuse and dependence. Alcoholism: Clinical and Experimental Research 20 (6): 1099-1 108.Naqvi, N.H.; Rudrauf, D.; Damasio, H. & Bechara, A. 2007. Damage to the insula disrupts addiction to cigarette smoking. Science 3 1 5 (58 1 1 ): 531 -34.Owen, A.M.; McMillan, K.M.; Laird, A.R. & Bullmore, E. 2005. N-back working memory paradigm: A meta-analysis ofnormative functional neuroimaging studies. Human Brain Mapping 25 ( 1 ): 46-59.Paulus, M.P.; Tapert, S.F. & Schuckit, M.A. 2005. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives ofGeneral Psychiatry 62 (7): 761-68.Pfefferbaum, A.; Desmond, J.E.; Galloway, C.; Menon, V.; Glover, G.H. & Sullivan, E.V. 200 1 . Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. Neuroimage 14 ( I Pt 1): 7-20.Pope, H.G., Jr. & Yurgelun-Todd, D. 1996. The residual cognitive effects of heavy marijuana use in college students. Journal ofthe American Medical Association 275 (7): 521-27.Pope, H.G., Jr.; Gruber, A.J.; Hudson, J.I.; Huestis, M.A. & Yurgelun-Todd, D. 200 I . Neuropsychological performance in long-term cannabis users. Archives ofGeneral Psychiatry 58 ( 10): 909-15.Pope, H.G., Jr.; Jacobs, A.; Mialet, J.P.; Yurgelun-Todd, D. & Gruber, S. 1997. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychotherapy and Psychosomatics 66 (4): 179-84.Robbins, T.W. 2000. Chemical neuromodulation of frontal-executive functions in humans and other animals. Experimental Brain Research 133 ( I ): 1 30-8.Rodriguez de Fonseca, F.; Ramos, J.A.; Bonnin, A. & Femandez-Ruiz, J.J. 1993. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4 (2): 1 35-38.Schwartz, R.H.; Gruenewald, P.J.; Klitzner, M. & Fedio, P. 1 989. Short-term memory impairment in cannabis-dependent adolescents. American Journal ofDiseases in Children 143 ( 1 0): 1 21 4-19.Schweinsburg, A.D.; Nagel, B.J. & Tapert, S.F. 2005. fMRI reveals alteration of spatial working memory networks across adolescent development. Journal of the International Neuropsychological Society I I (5): 63 1 -44.Schweinsburg, A.D.; Nagel, B.J.; Schweinsburg, B.C.; Park,A.; Theilmann, R.J. & Tapert, S.F. 2008. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging 163 ( I ): 40-5 1 .Schweinsburg, A.D.; Schweinsburg, B.C.; Cheung, E.H.; Brown, G.G.; Brown, S.A. & Tapert, S.F. 2005. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence 79: 201-10.Selemon, L.D. & Goldman-Rakic, P.S. 1 988. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior
parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience 8 ( I I ): 4049-68.Sneider, J.T.; Pope, H. G., Jr.; Silveri, M.M.; Simpson, N.S.; Gruber, S.A. & Yurgelun-Todd, D.A. 2006. Altered regional blood volume in chronic cannabis smokers. Experimental and Clinical Psychopharmacology 14 (4): 422-28.Solowij, N.; Stephens, R.S.; Roffman, R.A.; Babor, T.; Kadden, R.; Miller, M.; Christiansen, K.; McRee, B. & Vendetti, J. 2002. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal ofthe American Medical Association 287 (9): 1 1 23-3 1 .Spielberger, C .D.; Gorsuch, R.L. & Lushene, R.E. 1970. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.Tabachnick, B.G. & Fidell, L.S. 2007. Using Multivariate Statistics. Boston:Allyn and Bacon.Talairach, J. &Toumoux, P. 1988. Coplanar Stereotaxic Atlas ofthe Human Brain. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme.Tapert, S.F. ; Schweinsburg, A.D.; Drummond, S.P. ; Paulus, M.P.; Brown, S.A.; Yang, T.T. & Frank, L.R. 2007. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berlin) 194: 1 73-83.Tapert, S.F.; Schweinsburg,A.D.; Barlett, V.C.; Brown, G.G.; Brown, S.A.; Frank, L.R. & Meloy, M.J. 2004. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research 28 ( 10): 1577-86.Taper!, S.F.; Cheung, E.H.; Brown, G.G.; Frank, L.R.; Paulus, M.P.; Schweinsburg, A.D.; Meloy, M.J. & Brown, S.A. 2003. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives ofGeneral Psychiatry 60 (7): 727-35.Tapert, S.F.; Brown, G.G.; Kinderrnann, S.S.; Cheung, E.H.; Frank, L.R. & Brown, S.A. 200 1 . fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research 25 (2): 236-45.Thomas, K.M.; King, S.W.; Franzen, P.L.; Welsh, T.F.; Berkowitz, A.L.; Noll, D.C.; Birmaher, V. & Casey, B.J. 1999. A developmental functional MRI study of spatial working memory. Neuroimage 10 (3 Pt 1 ): 327-38.Uludag, K.; Dubowitz, D.J.; Yoder, E.J.; Restom, K.; Liu, T.T. & Buxton, R.B. 2004. Couplingofcerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage 23 ( I ): 1 48-55.Varma, V.K.; Malhotra,A.K.; Dang, R.; Das, K. & Nehra, R. 1988. Cannabis and cognitive functions: A prospective study. Drug and Alcohol Dependence 21 (2): 147-52.Verrico, C.D.; Jentsch, J.D. & Roth, R.H. 2003. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse 49 ( 1 ) : 61-66.Villares, J. 2007. Chronic use ofmarijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience 145 ( I): 323-34.Volz, K.G.; Schubotz, R.I. & von Cramon, D.Y. 2005. Variants of uncertainty in decision-making and their neural correlates. Brain Research Bulletin 67 (5): 403-12.Wager, T.D. & Smith, E.E. 2003. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience 3 (4): 255-74.Wager, T.D.; Sylvester, C.Y.; Lacey, S.C.; Nee, D.E.; Franklin, M. & Jonides, J. 2005. Common and unique components of response inhibition revealed by fMRI. Neuroimage 27 (2): 323-40.Ward, B.D. 2002. Deconvolution Analysis of FMRI Time Series Data. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin.Wechsler, D. 1 999. Manual for the Wechsler Abbreviated Scale ofIntelligence. San Antonio, TX: Psychological Corporation.Wechsler, D. 1997. Manualfor the Wechsler Adult Intelligence Scale-III.San Antonio, TX: Psychological Corporation.Wechsler, D. 1993. Manualfor the Wechsler Intelligence Scalefor Childrenlll. San Antonio, TX: Psychological Corporation.
Wise, S.P.; Boussaoud, D.; Johnson, P.B. & Caminiti, R. 1 997. Premotor and parietal cortex: Corticocortical connectivity and combinatorial computations. Annual Review ofNeuroscience 20: 25-42.Wong, E.C.; Luh, W.M.; Buxton, R.B. & Frank, L.R. 2000. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedingsof the International Society for Magnetic Resonance in Medicine8: 683.
Zurowski, B . ; Gostomzyk, J.; Gron, G.; Weller, R.; Schirrmeister, H.; Neumeier, B.; Spitzer, M.; Reske, S.N. & Walter, H. 2002. Dissociating a common working memory network from different neural substrates of phonological and spatial stimulus processing. Neuroimage 15 ( 1): 45-57.