Cannabis Study: How THC Affects Learning and Memory at Different Ages
This article originally appeared at https://www.leafly.com/news/science-tech/cannabis-study-thc-impact-on-memory-and-learning
A recent study in mice sparked eye-catching headlines like, “Memory Loss From Old Age Could Be Reversed By Smoking Marijuana.” The idea is alluring, especially given the toll cognitive decline takes as we age: instead of leaving you dazed and confused, THC might actually help restore cognitive function in older individuals.
While the study made interesting observations about how THC affects learning and memory in young vs. older mice, it didn’t involve smoking or cannabis consumption. What did the study find, how did it work, and what are the implications for future human research?
Cannabinoids and Aging: What Did We Already Know?
We knew three basic things going into this recent study. First, young mice have stronger learning and memory abilities than older mice—no surprise there. Second, giving young mice THC generally makes them perform worse on learning and memory tests. Third, the endocannabinoid system influences the progression of aging in the brain, and endocannabinoid levels in the brain decline with age.
Dr. Andras Bilkei-Gorzo, lead author of the recent study, explained the rationale for their experiments. “We had learned from previous work that decreased cannabinoid signaling accelerates brain aging. We asked whether enhancing cannabinoid system activity might slow down—or even reverse—normal cognitive decline that comes with aging.”
The idea was relatively straightforward. If age-related cognitive deficits are due, at least in part, to deficits in the endocannabinoid system, then perhaps exposure to a plant cannabinoid like THC might compensate for this. So, how did their experiments work?
THC, Memory, and Aging Study: Basic Findings and Summary
The study looked at behavioral measures of learning and memory in young vs. old mice. In each age group, some mice received a constant, daily dose of THC for 28 days, while others served as controls (they didn’t receive THC). After their 28-day treatment, their learning and memory abilities were assessed. There was no THC in their system during assessment. The question was how learning and memory were affected after chronic THC exposure.
It turned out that old mice responded differently to chronic THC compared to young mice. Old mice did better on learning and memory tests if they had a 28-day THC treatment beforehand. The behavior of old mice that had a chronic THC treatment looked like the behavior of young mice without a THC treatment.
There were also molecular changes in a brain area called the hippocampus that paralleled these behavioral changes. Basically, the brains of older mice that had received THC looked more like the brains of young mice without THC; there were more connections between neurons in the hippocampus. There were also some interesting genomic changes. In the THC-treated old mice, genes associated with plasticity and extended lifespan were turned up, while genes associated with age-related cognitive impairment were turned down.
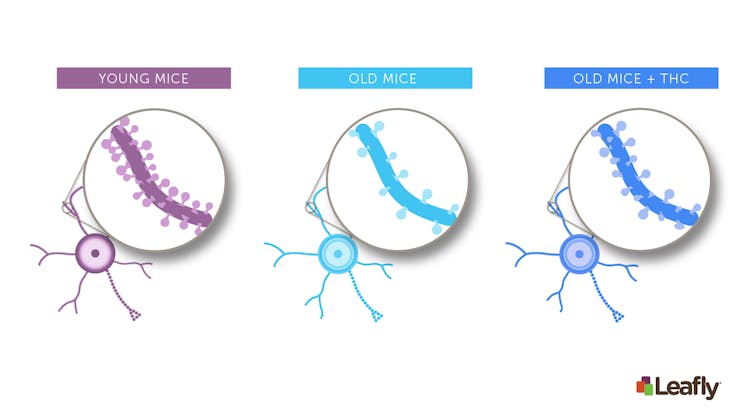
Figure 1: Chronic THC exposure in old mice can increase the number of connections between neurons in the brain. Brain cells often have structures called “spines.” Each spine marks a single connection between two brain cells. Compared to young mice (left), neurons in old-mice (middle) tend to have fewer spines. After chronic THC exposure (right), the brain cells of old mice often look more like those of young mice–they have more spines, and therefore more connections to other brain cells.
Cannabis and Aging: The Role of the Endocannabinoid System
As we age, our endocannabinoid system changes, including changes in CB1 receptor levels. CB1 is the receptor THC needs to activate for the classical effects of cannabis to be felt, and these receptor levels seem to generally decrease as we age. Perhaps chronic THC exposure in old mice restored cognitive function by increasing CB1 activation, compensating for the low overall levels of CB1 receptors. Using genetically engineered mice, researchers found evidence consistent with this idea.
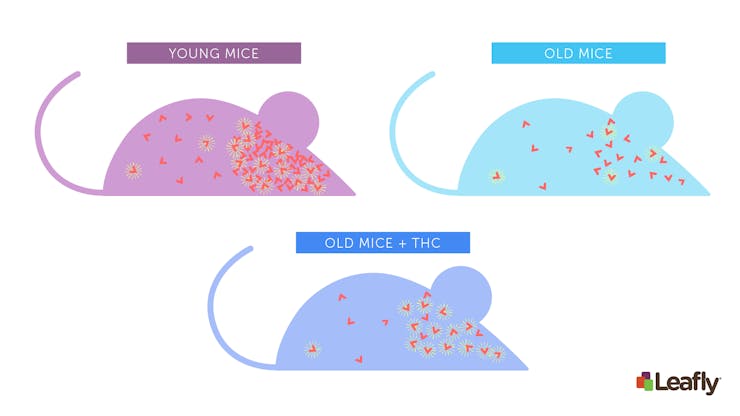
Figure 2: CB1 receptor levels decrease with age, and chronic THC exposure may compensate for this in old mice. Compared to young mice (top-left), old mice tend to have fewer CB1 receptors in their brain (top-right). Because THC activates CB1 receptors, chronic exposure to low-dose THC may compensate for this age-related change in the endocannabinoid system. Each red “V” represents a CB1 receptor. At any given time, some CB1 receptors may be activated (yellow lines) by cannabinoids, while others are not.
The broad takeaway from this study is that plant cannabinoids like THC can have very different effects on individuals depending on their age. These differences are likely due to age-related changes in the endocannabinoid system. Elevating cannabinoids levels may help compensate for some of these age-related changes.
Endocannabinoid System: Simple & Comprehensive Guide
These results should remind us to be cautious about generalizing the results of studies conducted in specific age groups. We should also hesitate to generalize the results of animal studies to humans, as there are important differences in how our bodies process biologically active compounds.
Study Caveat: Mice and Humans Metabolize Compounds Differently
The study’s title is, “A chronic low dose of THC restores cognitive function in old mice.” But how low was the “low dose” that the mice received?
In this study, mice were given 3 milligrams per kilogram (mg/kg) of body weight of THC per day for 28 consecutive days before learning and memory assessments. For a 150-pound person, that would be equivalent to about 204 milligrams of THC. Spread evenly throughout the day, that comes out to about 8.5 mg of THC every hour. A standard THC edible in a legal adult-use state is 10 mg, so the dose these mice were getting would be akin to taking an edible every hour of every day, for an entire month.
While that’s far from a low dose for a human, mice are a different story. Dr. Bilkei-Gorzo explained, “Humans are much more sensitive to psychoactive substances than rodents. The effective doses of anti-anxiety and anti-depression drugs is roughly 100 times higher in rodents compared to human patients. The same is true for THC—one needs a higher dose in rodents to see effects comparable to humans.”
Rodents and humans metabolize plant cannabinoids, pharmaceuticals, and other compounds at different rates, and sometimes in very different ways. That’s one big reason why we need to careful about jumping to conclusions about how animal studies will translate to humans.
Study Caveat: Cannabis Was Not Consumed, Pure THC Was Administered
The mice in this study were given pure THC through small devices surgically implanted under their skin. This allowed THC to be directly administered at a constant rate. Mice were not inhaling smoke or consuming cannabis products comparable to normal human consumption methods. This is another reason why we should be cautious when thinking about how cannabis consumption will affect learning and memory in older humans.
Questions for Future Research
Despite these caveats, the results of the study are intriguing and illustrate how plant cannabinoids like THC can have very different effects based on an individual’s age. These are likely due to age-related changes in the endocannabinoid system that unfold naturally over time. This points us to some important questions worth investigating in humans.
The first question is whether similar results would be seen in a human clinical trial. If so, would these results be seen after consumption of cannabis products through traditional consumption methods, or would older individuals need to acquire pure THC?
Thankfully, the researchers who conducted this animal study are designing experiments to investigate the effects of THC on elderly adults with mild cognitive impairment. “We are in the very beginning of the study design,” Dr. Bilkei-Gorzo explained. “The best-case scenario is that the clinical trial will start at the end of 2017 or beginning of 2018. The human trials will most likely use THC. This will allow us to precisely dose THC and compare the human results with the animal studies.”
While pure cannabinoid extracts are often used in human studies, there are legitimate reasons for why patients might respond differently to whole plant cannabis. “There are clear differences between cannabis and pure THC. Cannabis has the advantage of being better tolerated by patients, but pure THC can be precisely dosed.”
The Future of Medical Cannabis Research: Which Countries Will Lead the Way?
It’s also worth noting where the latest research is happening. This recent study was led by researchers at the University of Bonn, in Germany, in collaboration with scientists at Hebrew University in Israel. Israel has already established itself as the capital of medical cannabis research, while Germany and Canada are charging forward with federal legalization of medical and adult-use cannabis laws, respectively.
Meanwhile, federal prohibition in the United States means that medical cannabis research moves as slowly as possible. With the prospect of major budget cuts to government research agencies like the NIH looming, countries like Israel, Germany, and Canada may solidify themselves as the world’s medical cannabis research leaders, and be the first to reap the benefits.